This story originally appeared in the Breakthroughs Newsletter.
While the use of non-biological materials in science dates back thousands of years, Northwestern’s biomaterials labs are developing the next generation of materials in medicine, called supramolecular biomaterials — molecules designed in a way to mimic cell structures and functions of biological signaling.
Supramolecular biomaterials have the potential for many applications, including regenerative medicine, gene and drug delivery, tissue engineering and immunology.
Samuel Stupp, PhD, director of the Louis A. Simpson and Kimberly K. Querrey Institute for BioNanotechnology (SQI), is at the forefront of developing supramolecular biomaterials that can promote regeneration of tissues and organs, from bone and cartilage to muscle and brain tissues. Earlier this year, he received the Royal Society of Chemistry’s Soft Matter and Biophysical Chemistry Award for his research.
“What makes it exciting is you get to use cutting edge science — it’s new for everybody in the field — and it’s having an impact on lifespan and quality of life for people,” said Stupp, also a professor of Medicine in the Division of Endocrinology, Metabolism, and Molecular Medicine at Feinberg, of Materials Science and Engineering and Biomedical Engineering at the McCormick School of Engineering and of Chemistry at the Weinberg College of Arts and Sciences.
The field of supramolecular materials is based on supramolecular chemistry, a rapidly developing area in chemistry that studies the interactions among molecules and the way they can self-assemble into functional structures. The beginning of this field was recognized in 1987 when Donald J. Cram, Jean-Marie Lehn and Charles J. Pedersen received the Nobel Prize in Chemistry.
Part of Stupp’s work focuses on developing materials that mimic the nanoscale architecture of extracellular matrices surrounding mammalian cells, which have the ability to display biological signals that can interact with receptors and trigger signaling pathways. The signals from the artificial matrix can be used to amplify the potency of these pathways and also to promote cell proliferation and differentiation.
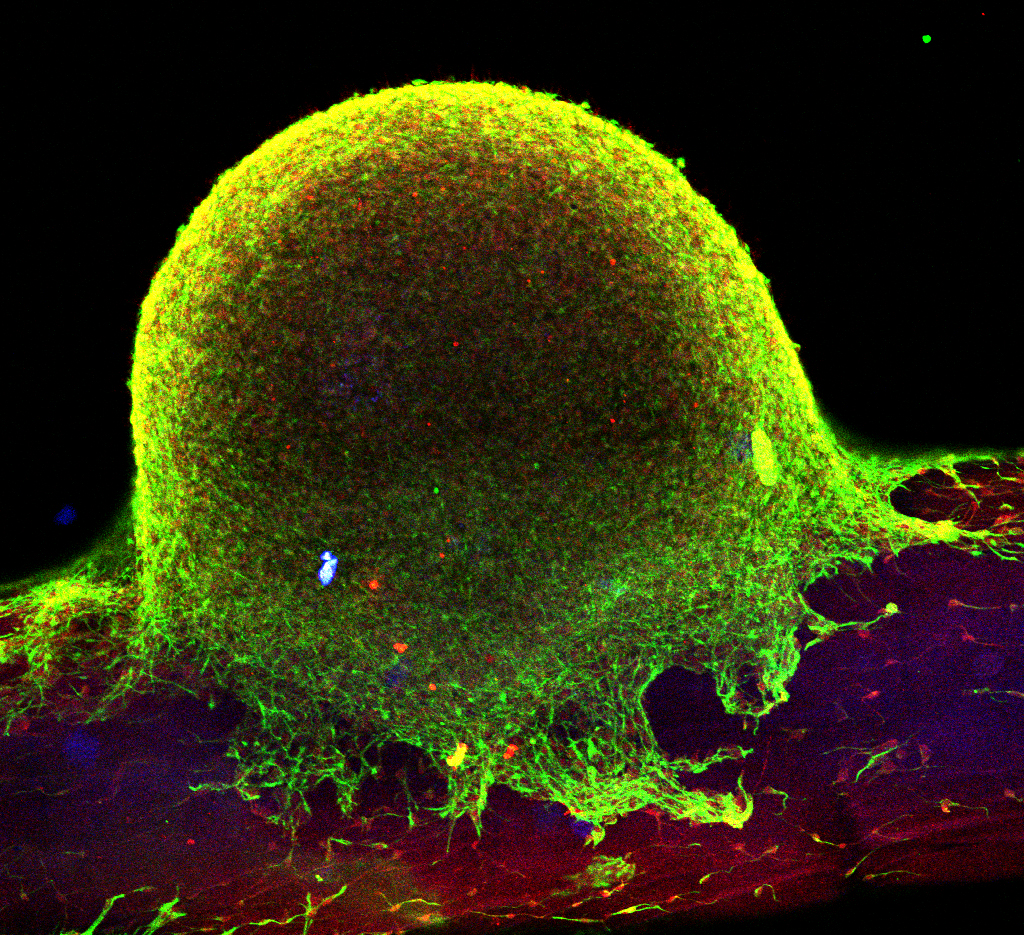
![Stupp Lab Photo[2]](/wp-content/uploads/2016/09/Stupp-Lab-Photo2.jpg)
The artificial nanofibers that Stupp has engineered resemble fibers of the cytoskeleton, collagen and extracellular filaments such as fibronectin and laminin fibers. They are built from a combination of amino acids, nucleic acids, lipids and sugars, which allows them to degrade into nutrients for cells without causing any harm. The scientists believe they can incorporate any biological signal in these nanofibers to achieve a specific therapeutic target.
This feature of the nanofibers has allowed Stupp to establish collaborations across the medical school including with John Kessler, MD, Ken and Ruth Davee Professor of Stem Cell Biology, on regenerating the nervous system in the spinal cord, Susan Quaggin, MD, chief of Nephrology, and Guillermo Oliver, PhD, Thomas D. Spies Professor of Lymphatic Metabolism, on targeting vascular regeneration, as well as Melina Kibbe, MD, adjunct professor of Surgery, on repairing vascular damage and targeting nanostructures to specific sites to reduce hemorrhage. Other successful collaborations with former Feinberg faculty involved cancer therapies, peripheral arterial disease and islet transplantation with Vincent Cryns, MD, currently at the University of Wisconsin, Douglas Losordo, MD, adjunct professor of Medicine, and Dixon Kaufman, MD, PhD, at the University of Wisconsin.
“One of the exciting ongoing projects is the possibility of using supramolecular materials to target plaque in arteries to reverse plaque formation,” Stupp said. This NIH-funded project involves collaborations with both Kibbe and Shad Thaxton, ’04 MD, ’07 PhD, assistant professor of Urology.
The nanomedicine research in Stupp’s lab currently advances to new unprecedented levels. In work published this year in Nature Materials, the group “demonstrated that the length of chemically identical nanofibers can determine whether mammalian cells survive and proliferate or not,” Stupp said.
In a paper published in Science earlier this year, Stupp’s lab described the development of new forms of the nanofibers that combines two types of polymers, those formed with covalent bonds and others formed with non-covalent bonds, also known as supramolecular polymers. The weaker covalent bonds in the new hybrid polymer gave it the ability to incorporate removable parts from the rigid stability of polymers with covalent bonds.
“These new unprecedented materials could be used as therapeutic agents to deliver drugs to cells over long periods of time or to affect cell behavior,” he said.
Stupp’s group used super resolution microscopy to illustrate that nanofibers are capable of rearranging their structures dynamically and could adapt to the receptor patterns on cells, work published recently in Nature Communications.
“This particular finding implies that highly bioactive nanoscale filaments will be possible in the future to address the most difficult regenerative and disease targets,” Stupp said.
They also discovered the nanofibers could interact with scaffolds on cell membranes to amplify BMP-2 signaling – critical in the regeneration of bone – by growth factors, in a paper published in Nanoletters.
Stupp is currently working in collaboration with Wellington Hsu, MD, Clifford C. Raisbeck, MD, Professor of Orthopaedic Surgery, and Erin Hsu, PhD, research assistant professor of Orthopaedic Surgery, on using these nanofibers in BMP-2 signaling in spinal fusion animal models.
“Innovation is essential to developing non-iterative solutions to clinical challenges,” Erin Hsu said. “Our work with the Stupp group has made such solutions a legitimate possibility. Because the platform for this technology is so broad, the prospect for its application in a wide variety of clinical settings is very exciting for us.”
In future work, Stupp’s group is developing new materials capable of displaying signals that can be turned on and off by adding certain molecules to cell cultures.
“This means that stem cells could be manipulated with one signal to promote their proliferation,” Stupp said. “That signal could be turned off when the cells are ready to differentiate and a new signal could be introduced for differentiation.”