An immune cell receptor called AXL has been linked to cardiac allograft vasculopathy (CAV), a thickening of vessel walls in transplanted hearts that occurs years after implantation, according to a Northwestern Medicine study published in the Journal of Heart and Lung Transplantation.
Inhibiting this receptor could be a viable therapy for the incurable condition, according to Edward Thorp, PhD, associate professor of Pathology in the Division of Experimental Pathology and senior author of the study.
“This is a promising target to ameliorate allograft rejection,” said Thorp, who is also a professor of Pediatrics. “This may not be specific to just hearts; this may also be seen in other organs, as well.”
Nearly half of heart transplant recipients develop CAV within 10 years. The walls of blood vessels supplying the heart thicken, narrowing vessels and restricting blood flow. This is especially dangerous because transplanted hearts are typically denervated — where a typical patient might feel chest pain, heart transplant recipients sometimes feel little until they experience severe symptoms, including heart attack or even sudden death.
“CAV is pretty much the number-one problem these days, because transplant physicians solved acute rejection with high doses of immunosuppression, but after five or 10 years, the graft begins to reject for other reasons,” Thorp said.
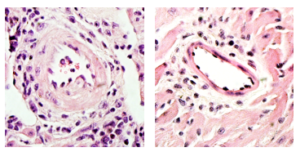
There are multiple contributors to CAV, but according to the study authors, one under-studied area is innate immunity. Previous studies from the Thorp laboratory had shown AXL receptors on innate immune cells play a role in modulating immune cell response in a variety of inflammatory conditions, so the investigators decided to examine human samples for elevated AXL activity.
They found that heart transplant recipients who developed CAV had markedly higher levels of AXL compared to healthy heart tissue. Taking the study into mouse models, they transplanted hearts into otherwise healthy mice, examining the immune response to the foreign organ.
“If you know the heart is not keeping them alive, we can look at the immune cell response to the organ without compromising the health of the mouse,” said Kristofor Glinton, PhD, a postdoctoral fellow in the Thorp laboratory and lead author of the study.
In these mice, higher levels of AXL meant a higher risk of CAV. Performing the same experiment in mice without AXL resulted in much lower rates of CAV, further confirming AXL’s influence.
“That told us AXL was playing a negative role, where it was creating an environment that was more inflammatory and not conducive to maintaining the graft long-term,” Glinton said.
Further investigation revealed that AXL is critical for inter-cell communication between immune cells and the blood vessel walls. Without AXL, immune cells were unable to promote the increased cell division that causes vessel wall thickening.
An AXL inhibitor had been previously developed to treat cancer, so Glinton applied this inhibitor to mice who received heart transplants and compared them to untreated controls. They found that mice treated with the AXL inhibitor maintained their transplants much longer than the untreated group.
For many patients who develop CAV, the only available treatment is another heart transplant. Extending the lifespan of a transplanted heart is only becoming more important, according to Glinton, as the population of patients requiring transplants is growing but supply is not keeping up with demand.
“The real goal is to maintain the transplant for as long as possible,” Glinton said. “This is even more important in juveniles with congenital heart defects who receive their first transplant when they are quite young, as they may require another heart when they’re still in their twenties or thirties.”
Inhibiting AXL may be one way to reduce the incidence of CAV and help patients maintain their heart transplants, freeing up valuable resources and improving health of transplant recipients.
“This possibility is really exciting, so anything that can prolong the lifespan of these grafts and improve quality of life makes the whole study worth it,” Glinton said.
This work was supported by the National Institutes grants R01HL139812 and R01HL122309, and American Heart Association and Enduring Hearts Foundation postdoctoral fellowship18POST33960228.