
Specially programmed stem cells demonstrated the potential to regenerate lost muscle mass in muscular dystrophy, according to a Northwestern Medicine study published in Nature Communications.
Elizabeth McNally, MD, PhD, the Elizabeth J. Ward Professor of Genetic Medicine, was a co-author on the study and Mattia Quattrocelli, PhD, a postdoctoral fellow in McNally’s lab, was one of the study’s co-senior authors.
Muscular dystrophy is a genetic disease characterized by the progressive loss of both skeletal and cardiac muscle mass. While there is no cure, physical therapy or medication can offer relief for symptoms, and recent advancements in induced pluripotent stem cells (iPSCs) have pointed to the possibility of future muscle regeneration therapies.
Past studies have shown that mouse-derived mesodermal iPSC-derived progenitor (MiP) stem cells can spur muscle regeneration in mice, with the unprecedented advantage of regenerating both heart and muscle tissue with the same stem cell type. However, the viability of human–based cells to perform similarly had been largely untested.
To investigate if human stem cells can effectively regenerate lost muscle tissue, the Northwestern Medicine scientists injected human MiP cells into mouse models, finding increased heart volume and improved muscle structure compared to controls with untreated muscle degeneration. When they later introduced a drug to downregulate the MiPs, the beneficial effects were reversed, bolstering the evidence that human MiPs have regenerative potential.
In addition, the study also explored ways to improve these stem cells’ ability to differentiate into both skeletal and cardiac muscle.
“While we can make stem cells differentiate into cardiac cells, making them differentiate into muscle cells has not been as easy,” said McNally, who is also the director of the Center for Genetic Medicine and a professor of Medicine in the Division of Cardiology and of Biochemistry and Molecular Genetics.
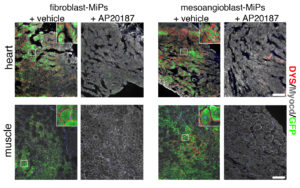
One possible solution is to use MiP cells created from skeletal muscle mesoangioblasts (MAB). The study also showed that these MAB-MiPs created more skeletal muscle cells when compared to MiPs derived from fibroblasts, a type of connective tissue cell. On the other hand, the capacity to generate cardiac muscle cells appeared comparable between the two.
However, treating fibroblast MiPs with microRNA cocktails showed even more promise, greatly improving the skeletal muscle differentiation of fibroblast MiPs, bringing them on par with MAB-MiPs.
In the future, these microRNA treatments could even be used to mobilize existing stem cells in addition to any newly injected cells, compounding the benefits of muscle regeneration therapy, according to the study.
“One of the most innovative aspects of this study is the identification of actionable molecules — the microRNA cocktails — to improve the innate efficiency of functional amelioration that human MiPs can impart on dystrophic muscle,” Quattrocelli said. “The next step will be to capitalize on these discoveries by improving safety and bringing this novel therapeutic option closer to clinical standards.”
McNally and Quattrocelli intend to continue exploring microRNA modulation and the use of MiPs in muscle regeneration, hopefully moving on to larger animal models as a proof-of-concept on the road to eventual patient therapy.
“If safety and efficacy are confirmed in larger models, this cell-based approach could theoretically help in combating many types of muscle degeneration in patients,” Quattrocelli said.






