Almost 20 years ago, Chad Mirkin, PhD, and his Northwestern University lab developed spherical nucleic acids, which have become the basis for more than 1,700 commercial products and medical applications including point-of-care medical diagnostic systems, brain tumor gene silencers and skin disorder treatments. The structure, composed of multiple strands of DNA and RNA densely arranged around a nanoparticle center, enters human cells better than any other form of nucleic acid.
Now, in a new paper published in Proceedings of the National Academy of Science, Mirkin and colleagues show that spherical nucleic acids can be used to regulate immune responses, a finding that may shift the way scientists think about developing therapeutic agents for a wide range of diseases.
“Spherical nucleic acids can be used to selectively stimulate or suppress the immune system in a very efficient manner,” said Mirkin, a professor in Medicine and in Chemistry at the Weinberg College of Arts and Sciences. “This is a complete paradigm shift in the field.”
Normal linear nucleic acids must be carried into cells using potentially toxic carriers such as viruses or polymers through a process called transfection. But the sphere-shaped nucleic acids are recognized by scavenger receptors on the surface of cells and shepherded into the cells on their own. They’re internalized through a process called endocytosis and end up in vesicles in the cells called endosomes. Inside and on the surface of these vesicles are toll-like receptors (TLRs), which are important immune response proteins.
“Engaging toll-like receptors allows you to regulate the immune system,” Mirkin said. “Not only are spherical nucleic acids able to enter cells better than conventional nucleic acids and without the need for transfection agents, they end up where you want them to be able to engage TLRs in a selective fashion.”

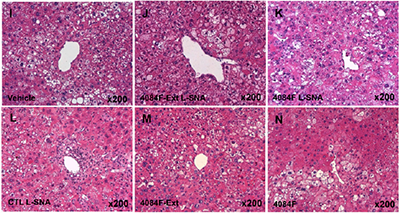
In the study, Mirkin’s group, in collaboration with AuraSense Therapeutics, found that organizing nucleic acids into spherical form led to significantly improved outcomes for two immunomodulatory therapies: One treated mice with lymphoma by stimulating the immune response and causing a selective elimination of cancer cells and corresponding increased life span, while the other treated liver inflammation called nonalcoholic steatohepatitis (NASH) by reducing the immune response.
“In principle, you can take advantage of the properties of spherical nucleic acids to hijack a person’s immune system, to train it to selectively eradicate cancer cells,” said Mirkin, who is a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. “In the case of diseases where you have inflammation, like rheumatoid arthritis, psoriasis or NASH, you can suppress the immune response.”
Mirkin’s team also showed that DNA and RNA molecules can be oriented on the surface of the particle in a way that maximizes their ability to engage cell receptors, leading to stronger therapeutic effects.
“Spherical nucleic acids can be hundreds to thousands of times more potent than linear nucleic acids delivered with transfection materials,” Mirkin said. “Next, we can use different nucleic acids to selectively engage different TLRs and begin to create a whole new platform for treating disease using immunomodulatory effects as a strategy. This will have very broad applications, affecting many diseases that can be improved by tweaking the immune system.”
This study is based on work supported by the Center for Cancer Nanotechnology Excellence initiative of the National Institutes of Health Award U54 CA151880 and the Defense Advanced Research Projects Agency Grant HR0011-13-2-0018.






