
Scientists have characterized how a motor protein helps cells crawl by using the latest advances in microscopy and also technology only available at Northwestern and a handful of other institutions worldwide, according to a study published in the Journal of Cell Biology.
Non-muscle myosin 2 is a motor protein involved in many cellular processes, most notably cell motility and contraction, which allows a cell to crawl and reorganize organelles within itself. To achieve this, non-muscle myosin 2 forms higher-order filaments within the cell, which then interact with actin cytoskeleton network, apply force and produce movement.
Until recently, the process of creating these higher order myosin structures from extremely tiny initial filaments, barely resolved via light microscopy, was not well understood, said Farida Korobova, PhD, research assistant professor of Cell and Developmental Biology, who was a co-author of the study.
In the current study, investigators employed the latest advancements in light and electron microscopy to observe these tiny filaments within a cell. By administering a drug cocktail that inhibited actin activity, investigators found that myosin filament formation was slowed, but not completely stalled.
Next, using a blue-light optogenetic technique, investigators recruited non-muscle myosin 2 to particular locations within a cell and found that local concentrations of the protein were sufficient to kick-start filament formation, according to the study.
“It was thought before that upstream molecular activators of myosin promote initial filament formation,” Korobova said. “But we found that the actin network actually serves as a modulator of the myosin concentration which drives the assembly of these myosin filaments.”
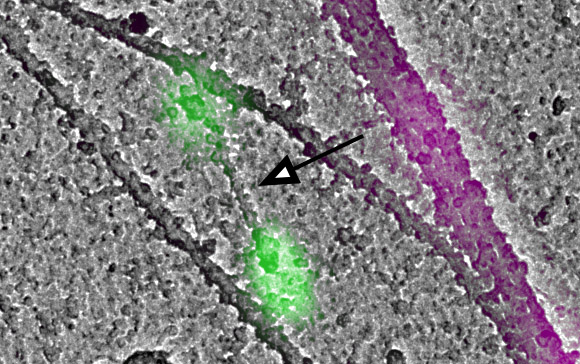
Additionally, investigators were able to characterize how the filaments organize themselves within a cell, insights which provide a better understanding of basic intracellular processes.
Taken together, the findings reveal the biophysical mechanisms regulating the assembly of non-muscle contractile structures that are ubiquitous throughout cell biology, Korobova said.
“These myosin motors are so universal in terms of cell types and motile cellular structures that studying them has a huge impact on many pathological processes like cancer and metastasis of the cells,” Korobova said. “This is a really fundamental mechanism which we have elucidated and described.”
Korobova and her collaborators will continue to study the proteins to further understand how the filaments form to move cells, she said.
“This was a true collaborative effort between Northwestern, Loyola and a few other Universities,” Korobova said. “I’m incredibly grateful we were able to use our expertise and techniques in light and electron microscopy to advance science.”
The study was supported by the Maximizing Investigators’ Research Award (MIRA R35) from the National Institute of General Medical Sciences, National Science Foundation CAREER Award #2000554, National Institute of Allergy and Infectious Disease grant # P01-AI02851 and the National Science Foundation Graduate Research Fellowship.






