Neural stem cells (NSCs) engineered by Northwestern Medicine investigators used in combination with the HER2 inhibitor drug tucatinib improved survival in mice with HER2-positive breast cancer brain metastases, according to findings published in Proceedings of the National Academy of Sciences.
The study, led by Maciej Lesniak, MD, chair and the Michael J. Marchese Professor of Neurosurgery, demonstrates the therapeutic utility of engineered NSCs for drug delivery to brain tumors and may lead to the development of novel and more efficient therapeutic options against HER2-positive breast cancer brain metastases.
Brain metastases are one of the main causes of mortality for patients with breast cancer. A lack of clinical trials coupled with the presence of the blood brain-barrier, which significantly decreases the efficacy of existing targeted therapies, have long stalled progress to improve patient outcomes.
The overexpression of HER2 is observed in about 30 percent of patients with breast cancer and is known to be associated with advanced disease and decreased overall survival. Additionally, about 50 percent of patients with overexpressed HER2-positive breast cancer will develop central nervous system metastases and are given an average survival rate of 11 to 18 months after diagnosis.
While chemotherapy drugs have improved outcomes for patients with primary breast cancer and patients with systemic metastases, more effective targeted therapies for patients with breast cancer metastases in the central nervous system are desperately needed.

“Systemic chemotherapeutic agents have a weak permeability to the brain, so we need to come up with innovative therapeutic regimens and delivery platforms that can improve clinical outcomes in patients with brain metastases,” said Alex Cordero-Casanovas, PhD, a postdoctoral fellow in the Lesniak laboratory and lead author of the study.
In the current study, Cordero-Casanovas and colleagues engineered human-derived NSCs to continuously secrete high amounts of antibodies that inhibit HER2 without compromising those cells’ stemness, migration and tumor-tropic properties.
After treating HER2-positive breast cancer cells in vitro with NSCs, the investigators found the secreted anti-HER2 antibodies blocked tumor cell proliferation by inhibiting the PI3K-Akt signaling pathway, which is essential for tumor cell growth and survival.
Next, the investigators developed HER2-positive breast cancer and brain metastases in vivo by injecting tumor cells in the carotid arteries of the mice, representing the multifocal nature of the disease in human patients, according to Cordero-Casanovas. The mice were then administered either NSC controls, NSC anti-HER2 NSC’s or placebo in combination with the HER2 inhibitor drug tucatinib, which was administered orally for twenty-four days.
Overall, mice that received anti-HER2 stem cells in combination with tucatinib showed a significant extended survival compared to mice treated with control cells or placebo with tucatinib.
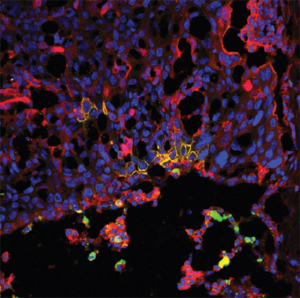
“Both the NSCs and tucatinib can cross the blood-brain barrier, so there are no issues with the permeability of the brain. Here we demonstrate that the combination of both agents were able to target HER2+ breast cancer cells in the brain and enhance survival in clinically relevant models of HER2+ breast cancer brain metastases” Cordero-Casanovas said.
For next steps, Cordero-Casanovas said the team is interested in studying the efficacy of this NSC-based therapeutic combination for the development of more efficient therapeutic options against HER2+ breast cancer brain metastases.
Co-authors of the study include Peng Zhang, PhD, research assistant professor of Neurological Surgery; Catalina Lee-Chang, PhD, assistant professor of Neurological Surgery; Jason Miska, PhD, assistant professor of Neurological Surgery; Irina Balyasnikova, PhD, associate professor of Neurological Surgery; and Atique Ahmed, PhD, associate professor of Neurological Surgery.
Lesniak, Zhang, Lee-Chang, Miska, Balyasnikova and Ahmed are members of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. Lesniak, Balyasnikova and Ahmed are also members of the Lou and Jean Malnati Brain Tumor Institute at the Lurie Cancer Center.
This work was supported by National Institutes of Health grants R35CA197725, R01NS093903, 1R01NS115955-01, R33NS101150, R01NS106379 and R37CA258426.