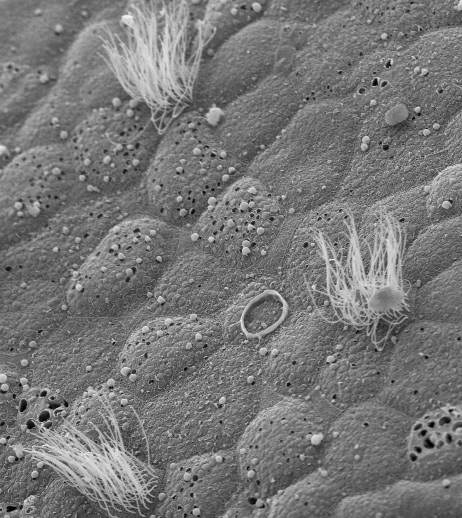
A new Northwestern Medicine study has found that cells with high numbers of centrioles migrate more quickly through layers of tissue, a process known as radial intercalation.
Many late-stage metastatic cancers have cells with abnormally high centriole counts, which means this process may represent an adaptation on the part of cancers in order to better proliferate, according to Brian Mitchell, PhD, associate professor of Cell and Developmental Biology and senior author of the study published in Current Biology.
“Essentially, more centrioles means more microtubules, which we believe gives the cell more rigidity,” said Mitchell, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. “Any migrating cell in cancer will have to get out of the site of primary tumorigenesis, and this could help.”
Radial intercalation is a process where cells move one layer “up,” pushing aside adjacent cells and thinning the new layer of tissue. It’s an important mechanism, especially in early development, according to Mitchell.
In the current study, investigators tracked this process in two different types of cells in frogs: multi-ciliated cells (MCCs), with cilia on their surface that beat in a coordinated manner to drive fluid flow, and usually have between 100 and 150 centrioles, and ionocytes, that have only two centrioles.
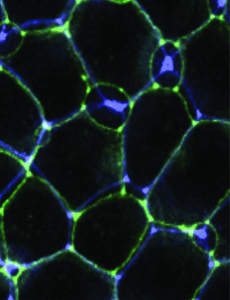
The authors found MCCs migrated about twice as fast through tissue when compared to ionocytes. When the investigators artificially decreased the number of centrioles in MCCs, they found migration was slowed, and when they increased in the number of centrioles, they found migration was faster than normal.
“It’s one thing when you break down a process and you make it go slower,” Mitchell said. “But when you’re making a process work better, that really implies an important contribution.”
How centrioles aid migration, according to Mitchell, is through the regulation of microtubules. More centrioles create more microtubules in the cell, which help make the cell more rigid.
In radial intercalation, these cells act like an icebreaker, punching through the layer of tissue — a mechanism that cancers appear to have adopted.
“Migrating cancer cells need to be able to penetrate barriers, so having more centrioles and therefore microtubules could allow them to break through layers of tissue more easily,” Mitchell said.
Blocking or disabling this mechanism could be a strategy to slow proliferation of cancer in the future, but more investigation is required, according to Mitchell.
“Hopefully, this inspires other groups to look at this issue and try to exploit this mechanism,” Mitchell said.
This work was supported by National Institutes of Health National Institute of General Medical Sciences grant R01GM119322.