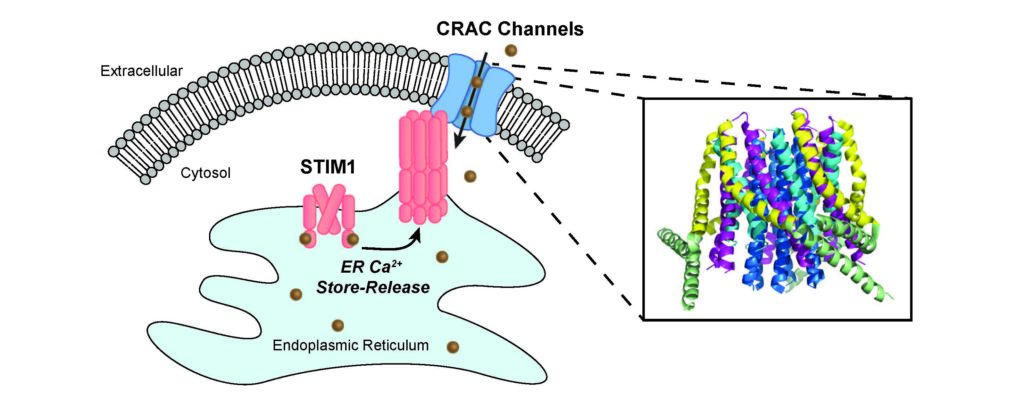
Northwestern Medicine scientists have identified the process that allows calcium channels to open and permit entry of calcium ions into cells, shedding light on how the channel operates and how mutations in the channel protein cause disease.
In the study, published in Nature Communications, the scientists identified the mechanism that keeps this particular calcium ion channel — called the Ca2+ release-activated Ca2+ (CRAC) channel — closed, as well as the movements in the channel pore that govern opening of the channel “gate.”
CRAC calcium channels are thought to exist in most, if not all, human cells, and play important roles in the activation of the immune system, muscle development and function and neuronal communication. When the channel pore opens, it allows calcium ions to flow into the cell, increasing the concentration of calcium in the cell and signaling functions such as gene transcription, proliferation and cell migration. A growing number of diseases are associated with abnormal CRAC channel function including immunodeficiency, muscular dystrophy and neurological diseases such as Alzheimer’s disease.
“Although the importance of CRAC channels for human health is now well recognized, how they operate at a molecular level remains poorly understood,” said senior author Murali Prakriya, PhD, associate professor of Pharmacology.

Megumi Yamashita, PhD, DDS, research assistant professor of Pharmacology, was the lead author of the study and Priscilla Yeung, graduate student in the Medical Scientist Training Program, also contributed to the paper.
“Megumi and Priscilla’s work reshapes our understanding of how the pores of these channels open in response to the stimulus, and specifically, it illuminates the molecular motions that govern the opening of the pore,” Prakriya said.
Using electrophysiology and microscopy techniques, the scientists observed that the CRAC channel’s gate uses a hydrophobic energy barrier that prevents charged calcium ions from passing through the pore in resting channels.
“In ion channels, the pore is usually water-friendly, so one way to close the pore is to have a oily, or hydrophobic, region that prevents the water-bound ions from going through — similar to the way that oil and water don’t mix. To open the pore, the hydrophobic region moves out of the way,” Prakriya said.
In addition to identifying the molecular structure that acts as the channel “gate,” the investigators also discovered that the pore-flanking domain of the channel rotates during channel opening. This allows the hydrophobic “gate” to move aside, thereby allowing the pore to become permeable to water and ions.
Using a supercomputer, collaborators at the University of Toronto ran molecular dynamic simulations, to show that this displacement of the hydrophobic gate eases an energy barrier, which increases water and ion occupancy in the pore when the channel opens.
The team also studied a human mutation in the calcium channel, which causes it to stay open, causing uncontrolled bleeding, muscle weakness and cognitive defects.
The investigators used the knowledge gained from the experimental and simulation studies to demonstrate that the human mutation disrupts the molecular stability of the hydrophobic gate, causing the pore to fill with water and letting ions travel through the channel.
“By understanding how the channel opens and closes, we can explain why human mutations cause abnormal channel activation,” Prakriya said. “In the long run, our hope is to use small molecule drugs that interact with the hydrophobic gate of the channel to manipulate its activity. This could correct defects in cell signaling and aid the quest for developing new therapies for immune, muscular and neurological diseases in which CRAC channels are involved.”
Next, Prakriya and his team plan to study the molecular rearrangements and signals that lead to the opening of the channel’s gate.
The research was funded by National Institutes of Health grants NS057499, GM114210, 5T32GM008382 and Canadian Institutes of Health Research grant MOP-130461.