Neonatal intestinal disorders that prevent infants from getting the nutrients they need may be caused by defects in the lysosomal system that occur before weaning, according to a new Northwestern Medicine study.
Lysosomes are cellular recycling centers responsible for breaking down all kinds of biological material. The study, published in PLOS Genetics, links lysosomal dysfunction with intestinal disorders for the first time, pointing to a previously unknown target for research and future therapies to help infants unable to absorb milk nutrients and gain weight, a diagnosis called failure to thrive.
“This finding highlights the critical role the lysosomal system plays in neonatal digestion,” said principal author of the study Jaime García-Añoveros, PhD, associate professor in Anesthesiology, Neurology and Physiology. “We suspect that many neonatal intestinal pathologies – leading causes of infant mortality worldwide – are not appropriately understood and thus treated. The study suggests looking for lysosomal defects in these pathologies.”
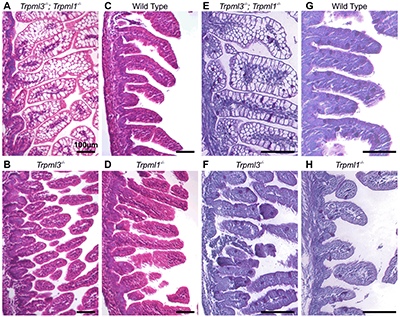
The investigators found that mouse models lacking certain lysosomal proteins developed an intestinal disorder that caused diarrhea and delayed growth between birth and weaning. For all mammals, including humans, intestinal digestion during this nursing period is very different from adult digestion.

“Normally food proteins break down in the stomach, and the resulting amino acids are absorbed in the intestine,” García-Añoveros said. “But the stomach is not acidic yet in a nursing baby.”
Instead, proteins reach the intestine undigested in infants. There, cells lining the small intestine called enterocytes degrade the proteins and absorb the amino acids using special lysosomes with digestive enzymes.
In the study, mice that lacked two lysosomal proteins normally expressed by enterocytes experienced failure to thrive symptoms throughout the nursing period. The finding suggests that infants with lysosome disorders may be affected with intestinal disorders.
Should further research identify this sort of lysosomal dysfunction in humans, infant formulas with proteins already broken down into amino acids could be a simple therapy, said García-Añoveros, who is a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
“Right now I don’t think physicians are thinking of the lysosomal system as particularly important in the intestines,” he said. “But we have shown that they are very prominent in digestion during this period of an infant’s life.
This research was funded by National Institutes of Health (NIH) grants RO1 NS044363 from the National Institute of Neurological Disorders and Stroke, F31 DC010529 from the National Institute on Deafness and Other Communication Disorders and T32 NS041234.






