Scientists may be a step closer to understanding the biological basis of the aging process: A Northwestern Medicine study has linked aging at the cellular level to overall human aging through a molecular interaction involving two proteins and chromosome ends called telomeres.
Telomeres are repetitive sequences of DNA found at the ends of chromosomes, protecting them during cell division. A cell replicates about 40 to 60 times, and each time the telomere gets a bit shorter. Eventually, it’s so short that the cell can no longer divide.
“The progressive shortening of telomeres is a readout of a cell’s age,” said Steven Kosak, PhD, assistant professor in Cell and Molecular Biology and a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. “They also protect the chromosome from being recognized as damaged, broken DNA.”
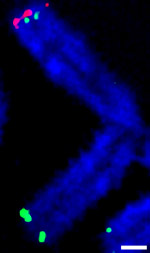
Telomeres do this by circling back on themselves and forming a loop, called a t-loop. In the study, published in Nature Communications, principal investigator Kosak and first author Ashley Wood, PhD, research assistant professor in Cell and Molecular Biology, found that chromosome ends can circle back not only into telomeres, but also directly into the chromosomes. At these sites, they showed that the telomere binding protein TRF2 interacts with another protein called lamin A/C to build the protective loop into the chromosome, which the scientists called an interstitial t-loop.
Interestingly, the gene that codes for lamin A/C is mutated in patients with Hutchinson Gilford Progeria Syndrome, a rare disorder that causes premature aging and death in the mid-teens.

“How this protein can lead to human aging has been poorly understood. We thought it might be responsible for forming the loops,” Kosak said. “Sure enough, when we knocked down lamin A/C in cells, TRF2 didn’t bind to these interstitial t-looping sites anymore.”
Patients with Progeria Syndrome have a shortened form of lamin A/C called progerin, which mimics the effect of reduced lamin A/C: failing to properly facilitate TRF2 binding. Previous studies have also detected increased progerin in elderly people.
Altogether, the study provides evidence that lamin A/C and TRF2 work together at sites of the newly-identified interstitial t-loops to maintain the protective effects of telomeres. When the process is upset – through the removal of either protein – cellular aging and human aging accelerate.
These results open up a new avenue for research on aging and its symptoms.
“If aging can be characterized as a disruption to normal physiology, we may be able to ameliorate some of the more debilitating – and medically expensive – aspects of what some consider a disease,” Kosak said.
This work was supported by an Ellison Medical Foundation New Scholar Award and National Institutes of Health (NIH) New Innovator Award DP2 OD008717-01.






