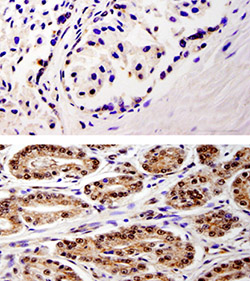
Elevated levels of a protein associated with cell cycle regulation may allow physicians to identify prostate cancer at its earliest stages.
Debabrata Chakravarti, PhD, professor of Obstetrics and Gynecology-Reproductive Biology Research, led the Northwestern Medicine study, which revealed the surprising role for WDR5.
“Our results showed that the overexpression of this protein may be necessary for the initiation, maintenance and growth of prostate cancer,” said Chakravarti, a member the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. “If WDR5 is in fact a driver protein, we may also be able to develop a compound that would block a critical interaction and stymie the disease.”
The findings were published in Molecular Cell.
Prostate cancer is the second-leading cause of cancer deaths in North American men. In the United States, more than 225,000 are expected to be diagnosed with the disease this year and nearly 30,000 will die. Unlike breast cancer, the second-most common malignancy in American women, very few genetic abnormalities have been linked to the disease.
What scientists do know is that prostate cancer is stimulated by testosterone, a form of the male hormone androgen. The most common way to determine if a person has the malignancy is to perform a blood test for prostate specific antigen (PSA). The higher a man’s PSA level, the more likely it is that he has prostate cancer. However, false positives and negatives can occur.
“PSA is an outcome-based measure showing the possible presence of prostate cancer. We wanted to focus our efforts on the earliest events that may promote its development,” Chakravarti said. “Increased understanding of epigenetics indicates that the epigenome, or chemical changes to an organism’s DNA and its associated proteins called histones, is as important as or possibly even more important than our DNA.”

Unlike the genome, the epigenome can be dynamically altered by environmental conditions. What Chakravarti discovered is that WDR5 integrates androgen activity into the epigenome to stimulate prostate cancer growth.
“What this epigenetic change does is brings more dancers to the floor. One epigenetic change is helping establish another and together they are regulating genes that contribute to cancer growth,” Chakravarti said.
Scientists analyzed nearly 100 prostate samples and found WDR5 to be present at a very low level in normal tissue. However, the amount rises when prostate cancer is present.
To verify the role the protein plays, Chakravarti and colleagues removed WDR5 from prostate cancer cells in a petri dish. The result was that the cancer couldn’t grow, suggesting the possibility that a drug which could dampen this activity may be beneficial as treatment.
The work was done in collaboration with Northwestern Medicine colleagues Jian-Jun Wei, MD, and Grant Barish, MD, as well as Ishwar Radhakrishnan, PhD, professor of Molecular Biosciences.
“The finding is exciting because it tells us that the protein is directly involved in the prostate cancer process,” Chakravarti said. “If the upregualtion of WDR5 can be detected, prostate cancer will be revealed at its initial stages. A drug that could stop the epigenetic interaction would slow disease progression.”
Chakravarti’s laboratory is currently analyzing such possibilities.
The work is supported by National Cancer Institute grant RO1 CA133755, National Institute of Child Health and Human Development grant PO1 HD 57877 and the Robert H. Lurie Comprehensive Cancer Center.
Ji-Young Kim, lead author, Aurimas Vinckevicius and J. Brandon Parker also contributed to this study.






