Northwestern Medicine scientists have uncovered a critical protein in the formation of normal platelets, a finding that could someday help turn large-scale production of the blood product into a reality.
“What we found is a novel mechanism for how platelet precursors are generated,” said principal investigator Seth Corey, MD, MPH, professor in pediatrics-hematology, oncology, and stem cell transplantation and cell and molecular biology. “The loss of CIP4, a protein that bridges the barrier of these cells with their skeleton-like infrastructure, causes a defect in platelet production.”
The discovery was recently published in the journal Blood.
One of three major components of blood – red cells shuttle oxygen while white cells fight infection – platelets are responsible for the thinning and clotting that keep us alive.
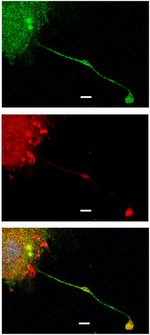
They are derived from megakaryocytes, specialized cells that replicate their DNA but do not divide, meaning they grow in size unlike any other cell in the body. After a number of cell cycles, the megakaryocyte implodes, releasing finger-like projections known as pro-platelets, or platelet precursors. Each megakaryocyte can account for thousands of platelets.
Corey’s lab confirmed in animal models and cell lines that a loss of CIP4 resulted in a decreased number of pro-platelet projections, causing a decreased number of platelets.
“This shortage either represents a production defect or a destruction problem and we think in this case, it is the former,” said Corey, a member of the Ann & Robert H. Lurie Children’s Hospital of Chicago Research Center. “Somehow the loss of CIP4 is preventing the production of an optimal number of normal platelets.”
Low platelet counts increase the risk of hemorrhage, or internal bleeding.
For her role in the project, Yolande Chen, MD, first author of the paper, received an American Heart Association (AHA) postdoctoral fellowship to study platelet production and thrombocytopenia – low platelet counts.
The lab’s research was funded by the National Institutes of Health (NIH) and the AHA.
Platelet Shortages
Because individual platelets only live for about 10 days, the human body continually renews its supply by producing new platelets in the bone marrow.

When the body is unable to keep up with demand – as can be the case during surgery, chemotherapy, or bone marrow transplantation – a platelet transfusion is done.
Donated platelets are always in short supply, but Corey believes the quest to efficiently produce them in tissue culture may be nearing a successful conclusion.
“The more we understand about how platelets are produced, the closer we can get to engineering an artificial system to create ones that are healthy and tolerated by all,” said Corey, a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. “It’s a soup to nuts kind of thing where we have to engineer the process starting with the stem cell and making an efficient megakaryocyte that will result in universal donor platelets.”
ASH Award
The American Society of Hematology (ASH) recently launched an innovative program to support the research of scientists in the field. In August, Corey’s lab received one of 12 new ASH Bridge Grants for his proposal.
“This grant will cover a project on why people get myelodysplastic syndrome (MDS), formerly known as pre-leukemia, and then develop leukemia” Corey said. “The funding is for one year and will permit us to perform some expensive experiments to gather data and get a grant funded in the future.”
As a pediatrician at Lurie Children’s, Corey treats patients with bone marrow failure. Unfortunately, many of these patients go on to develop MDS. Using genetic, biochemical, and mathematical modeling, Corey and his co-investigators are continuing to identify the misinformation and forces that cause disease transformation. The goal is to identify the period of vulnerability, identify early sensors, and intervene before the disease becomes malignant.






