What started as a “random” dot under fluorescent microscopy more than five years ago is today the focal point of a paper in a major publication.
Discovered by Brian Mitchell, PhD, assistant professor of cell and molecular biology, the protein CCDC78 marks a significant advancement in understanding the formation of multi-ciliated cells.
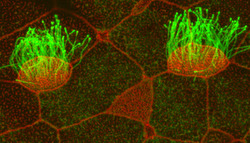
Cilia, which resemble hair-like projections, are found in cells lining the trachea, where their wave-like motion sweeps mucous away from the lungs; in the fallopian tubes, where they are responsible for moving eggs from the ovary to the uterus; and in the brain, where they drive cerebral spinal fluid through the ventricles.
The body’s multi-ciliated cells represent an interesting biological variation in that they generate more than 100 centrioles, an organelle crucial for cell division that typically numbers just two. This increase in centrioles depends on the deuterosome, an uncharacterized structure thought to be the “factory” which churns them out.
Featured in the October 14 issue of Developmental Cell, Mitchell found that the dot he noticed a half-decade ago is actually the precise location of centriole amplification.
“Despite the many advances in molecular and cell biology over the past 50 years, somehow the deuterosome had remained molecularly uncharacterized,” Mitchell said. “Our work describes for the first time the mechanism that is required to generate hundreds of centrioles, which allow for the proper development of ciliated tissues.”
No matter the location, the multi-ciliated tissues of humans are remarkably similar and share many features with the multi-ciliated tissue found on the surface of developing Xenopus (frog) embryos.
Because of this, the animal model offers a “perfect” system for understanding the molecular processes required to form a functional multi-ciliated tissue.
Since centriole duplication is typically one of the first steps in the cell cycle and is critical to proper cell division, Mitchell’s lab remains interested in the creation of organelles in cells that make many cilia. Understanding how nature has uncoupled centriole duplication from the cell cycle could lead to insights into how the process goes awry during cancer development.
“Beginning the process of defining the mechanism allows us to then think about the difference between how these multi-ciliated cells make their centrioles versus how a dividing cell makes its centrioles,” said Mitchell, a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. “We now have the players and tools to dig deeper into the biochemistry of how these proteins are regulated.”
This research was supported by the National Institutes of Health through National Institute of General Medical Sciences grant R01GM089970, a pilot grant from the Northwestern University Skin Disease Research Center, an American Recovery and Reinvestment act grant, and a postdoctoral fellowship grant from the American Heart Association.






