A new Northwestern Medicine study shows that a protein called POP1 prevents severe inflammation and, potentially, diseases caused by excessive inflammatory responses.
Autoinflammatory diseases occur when inflammation, the body’s reaction to infections and injuries, is over-activated. The new paper, published in Immunity, describes how POP1 blocks a pro-inflammatory mediator called IL-1b.
“Inflammation is controlled by a complex network of regulators that is still poorly understood,” said senior author Christian Stehlik, PhD, John P. Gallagher Research Professor of Rheumatology. “Here, we describe a novel inhibitor of the inflammatory response.”
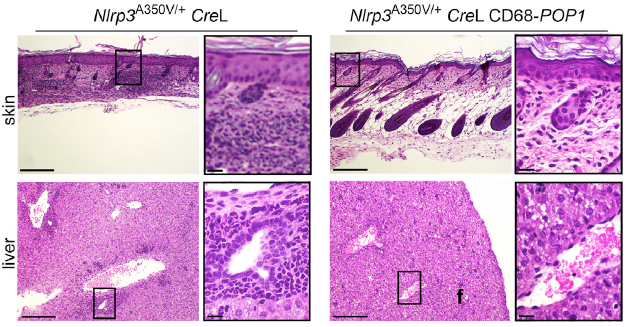
Stehlik worked with co-senior author Andrea Dorfleutner, PhD, research assistant professor in Medicine-Rheumatology, and a team headed by postdoctoral fellow Lucia de Almeida, PhD. They found that mice expressing POP1 were protected from systemic inflammation. Those mice also recovered more quickly than controls from inflammatory diseases, including peritonitis, sepsis and cryopyrinopathy.
Interestingly, the scientists also discovered that human patients with cryopyrinopathy and sepsis have less POP1 in their immune cells than others.

“Based on this finding, we developed a proof-of-principle drug based on POP1 and administered this treatment for peritonitis in a mouse model,” Dorfleutner said. “We found significantly reduced inflammation.”
The scientists believe that treatments based on POP1 could improve many inflammatory diseases, not just the ones tested in this study.
“A better understanding of the molecular events that contribute to autoinflammatory diseases is critical for the development of novel therapies that may benefit patients in the future,” said Stehlik, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. “Understanding the critical role of POP1 in inflammation leads us to believe that novel POP1-based therapies may be applicable in humans.”
In a previous study, Stehlik and colleagues showed how another protein in the POP family, POP3, inhibits the immune response to virus infection. Unlike POP1, POP3 did not affect autoinflammatory disease. POP proteins are unique in their function to regulate specific aspects of the immune response.
The Stehlik lab worked in collaboration with Harris Perlman, PhD, Solovy/Arthritis Research Society Professor in Medicine-Rheumatology and Alexander Misharin, MD, PhD, research assistant professor in Medicine-Pulmonary, at Feinberg, as well as David Greaves, PhD, at the University of Oxford and Hal Hoffmann, MD, at the University of California, San Diego.
This research was supported by National Institutes of Health grants GM071723, HL097183, AI092490, AI082406, AI099009, AI120625, AR064349, AR057532, AR066739, AR050250, AR054796, AI092490, HL108795, AR061593 and AI52430; Cancer Center Support Grant CA060553; Skin Disease Research Center grant AR057216; American Heart Association grant 13GRNT17110117; an ATS/Scleroderma Foundation; and British Heart Foundation grant RG/10/15/28578.






