
Northwestern Medicine scientists have discovered a clever evolutionary quirk in multi-ciliated cells, which help drive fluid flow in a variety of body systems.
The findings, published in Nature Cell Biology, suggest that small structures called deuterosomes are not as vital as previously thought, contributing to a new understanding of how these cells develop.
“Our lab, as well as many others, has been trying to identify novel deuterosome proteins in order to understand the process, but it now looks like that might not be the right direction to be focusing on,” said Brian Mitchell, PhD, associate professor of Cell and Developmental Biology and a co-author of the study.
Multi-ciliated cells (MCCs) use small microscopic “hairs” called cilia, beating in a coordinated manner to move materials and fluid around the body, including the movement of mucus and debris out of lungs or to circulate cerebral spinal fluid in the brain. These cilia sprout from centrioles, small organelles on the surface of MCCs. Typical cells usually have just two centrioles and during cell division these centrioles are duplicated once, resulting in a daughter cell with two centrioles.
In MCCs, however, there are dozens or hundreds of centrioles to support the many cilia needed to propel fluid, necessitating a different parent organelle, according to Mitchell.
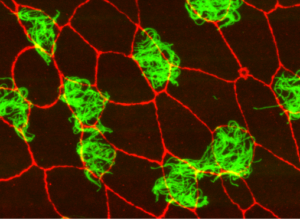
“There’s this massive increase in centrioles, and this could not be as easily templated off of existing centrioles,” said Mitchell, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. “Many years ago, it was discovered that MCCs contained a novel structure — the deuterosome — from which most of the centrioles nucleated off.”
Recent work in the Mitchell laboratory has suggested important proteins travel to the deuterosome and regulate centriole formation, furthering suspicion that these deuterosomes are crucial for creating centrioles and therefore functional cilia.
In the current study, Mitchell and his colleagues created MCCs without deuterosomes or centrioles, to explore how the cells were affected. Surprisingly, they weren’t.
Instead, the cells created the correct amount of centrioles and cilia, without parent deuterosomes or centrioles.
“These cells were still able to find a way to nucleate new centrioles, with nothing to template them from,” Mitchell said.
The discovery of this secondary centriole production mechanism underscores their importance, and not just in humans — cilia serve crucial functions in many animals, such as to propel aquatic animals or facilitate feeding.
“There must be a lot of evolutionary pressure to make sure that MCCs can generate many centrioles,” Mitchell said. “It is likely that this back-up mechanism is the result of this pressure and the overall importance of these cells to survival.”
Mitchell said he believes this discovery will prompt some second thoughts among fellow scientists studying deuterosomes, who may shift from examining deuterosomes to other aspects of cilia or centriole formation.
“I think this paper will change the direction of numerous labs as they refocus on the problem, knowing that these key structures are not essential to the process,” Mitchell said.
This work was supported by National Institutes of Health grants R01GM089970, R01GM114119 and R01GM133897 and the American Cancer Society Scholar grant RSG-16-156-01.






