
Northwestern Medicine scientists have discovered increased immune cell activity in Merkel cell carcinoma tumors, which could help predict treatment response in patients and inform the development of new targeted therapies, according to findings published in the journal Cancer Discovery.
“We hope to use this as a biomarker to discover why some patients respond, so they can get the best treatment as soon as possible but also to inspire new therapies that we can use to get them to respond to,” said Jaehyuk Choi, MD, PhD, the Jack W. Graffin Professor of Dermatology and of Biochemistry and Molecular Genetics.
Merkel cell carcinoma is an uncommon and aggressive type of skin cancer with fewer than 3,000 cases diagnosed in the U.S. each year, according to the American Cancer Society.
The cancer has the appearance of Merkel cells, which are located at the base of the epidermis and connected to nerve endings in the skin. A recently discovered virus, called Merkel cell polyomavirus, is suspected to cause a majority of cases, however people can also develop the cancer from excessive sun exposure or by having a weakened immune system.
Immune checkpoint blockade therapy is the current standard of care, but only half of patients will respond to the therapy. Why other patients experience treatment resistance has remained poorly understood.
To identify potential predictive biomarkers of treatment response, Choi’s team used a combination of single-cell RNA-sequencing and spatial transcriptomics to analyze tumor and blood samples from 116 patients diagnosed with Merkel cell carcinoma.
“These techniques allowed us to examine the gene expression of each individual cell within the patient’s tumor and to determine how these cells interact with each other within the tumor microenvironment,” said Zachary Reinstein, a student in the Medical Scientist Training Program (MSTP) and lead author of the study.
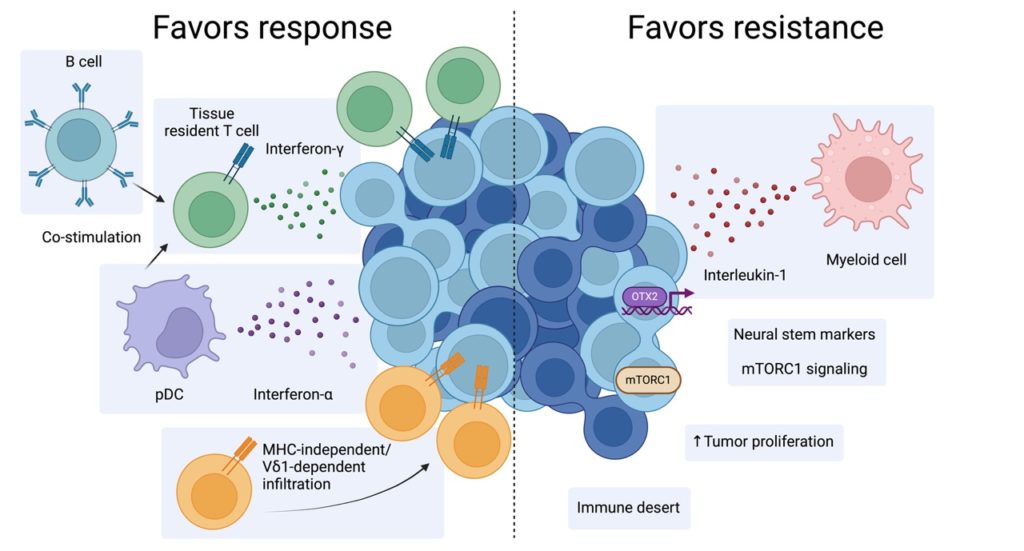
Using these techniques, the scientists discovered that interferon gamma, an immune signaling cell, drives treatment response and resistance in the tumor microenvironment. Interferon gamma is a cytokine that alerts the immune system when healthy cells have been infected by a virus or cancer.
“We could actually track in real time the anti-tumor immune response in the patients who responded to treatment. This has enabled us to see how these tumors are being attacked in patients who respond to treatment and how they create defenses when they’re not responding,” said Choi, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
This anti-tumor immune response is driven by an increase of specialized CD8 T-cells called tissue resident CD8 T-cells, which are found in skin tissue. The scientists also identified another subset of immune cells, called V-delta-1-gamma-delta T-cells, which target the tumor regardless of its ability to respond to interferon gamma.

“Skin has very important defense mechanisms to target skin cancers and what we think is that these cells are the primary defense against the tumor,” Choi said.
The findings may inform the development of new targeted therapies for Merkel cell carcinoma, as well as for other types of cancer, according to Choi.
“Certainly, we’re going to use these findings to inspire new treatments for patients with this cancer. One of the other things interested in thinking about is how we can use this as a model system to better understand why all cancers respond or don’t respond to immunotherapy,” Choi said.
Co-authors include Sunandana Chandra, MD, MS, associate professor of Medicine in the Division of Hematology and Oncology and Jeffrey Sosman, MD, professor of Medicine in the Division of Hematology and Oncology.
Chandra and Sosman are also members of the Lurie Cancer Center.
This work was supported by the Melanoma and Skin Cancer Center of Excellence, the Barry S. Greene Fund and the Moffitt Distinguished Scholar Award, the National Cancer Institute grant T32 CA009560 and F30CA278298, the 2019 AOA Carolyn E Kuckein Fellowship, the V Foundation for Cancer Research grant T2021-019, the Bakewell Foundation, the National Institutes of Health grants DP2 OD024475-01, R35 GM144083, and the Leukemia and Lymphoma Society grant 1377-21.






