This story was published in the October 2023 issue of the Breakthroughs newsletter.
Sex is a major determinant in disease prevalence and treatment response, caused by a vast number of genetic differences between men and women. However, the inclusion of both sexes in clinical and scientific research had not been mandated by federal law until nearly the turn of the century.
In 1993, Congress wrote the Women and Minorities as Subjects in Clinical Research law into the National Institutes of Health (NIH) Revitalization Act of 1993, which requires the NIH to include cisgender women and racial and ethnic minority groups as subjects in all clinical trials, and that these trials are designed to allow scientists to determine whether the variables being studied affect these groups differently than other participants.
In response, significant strides in sex-based research have been made over the last three decades, but according to Barbara Stranger, PhD, associate professor of Pharmacology, the work continues.

“The simplest thing we [scientists] can do to consider sex as a potential influence on any kind of trait, disease or relationship is to disaggregate our data by male and female study participants. The problem with not doing these kinds of analyses means that you miss the opportunity for a more personalized-by-sex answer to whatever question you may have,” Stranger said.
In a recent editorial published in the journal Cell, Stranger argues that despite the success of genome-wide association studies which explore how genetic variation contributes to the manifestation of complex traits or diseases, the use of data to investigate sex differences in the underlying mechanisms has been scarce.
In her editorial, which outlines best practices for investigators studying and testing for sex-dependent genetic effects, Stranger also asserts that designating sex as a biological variable in clinical and scientific research will accelerate the understanding of disease risk and biology in both sexes and improve precision medicine and equitable care.
“We won’t know if we don’t look… and this really has potential to discover new knowledge and help contribute to a more personalized approach to many aspects of medicine,” Stranger said.
A New Wave of Research
Feinberg investigators across specialties are studying how sex-based differences impact various aspects of health and disease, including behavior, cognitive function in response to stress, and the manifestation of pain.
In the laboratory of Julia Cox, PhD, research assistant professor of Neuroscience, her research aims to understand the neural mechanisms that influence decision-making and how these mechanisms may differ between each sex.

In a recent study published in Nature Neuroscience, Cox’s team studied the activation of a subset of neurons in mice while the mice were tasked with choosing a lever to press in the hopes of receiving a reward.
By performing two different experiments, Cox found that the mice’s motivation to engage in the task was influenced by “action value,” or how likely they were to receive a reward for an action based on their own past experiences. Furthermore, Cox’s team observed that this this “action value” behavior was demonstrated more strongly in the female mice than the male mice.
Replicated experiments involving human participants had confirmed their findings — female participants were slower to reengage in the task based on what happened in previous trials compared to male participants.
“I think that more broadly, when studying sex differences in laboratory animals, even if the behavioral differences don’t always end up translating to humans, it’s possible that some of the differences in the neural circuits still are consistent across species,” Cox said.
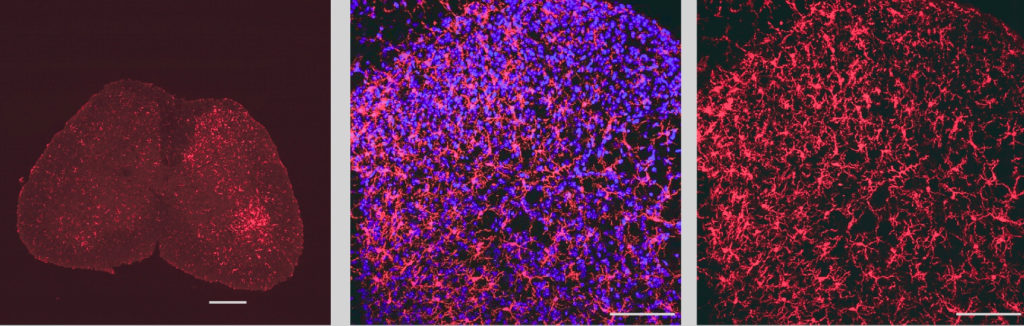
Other Feinberg investigators have also identified sex-based differences at the intercellular level. In a study led by Murali Prakriya, PhD, the Magerstadt Professor of Pharmacology, and published in Sciences Advances, investigators found that specific calcium channels in microglia — the primary immune cells of the brain — regulate sex differences in the functioning of immune cells in response to neuroinflammation and neuropathic pain.

More than a decade ago, Prakriya’s laboratory discovered a class of calcium channels called Orai1 channels. In the current study, his team sought to determine what role Orai1 plays a role in microglial activation and neuropathic pain following nerve injury.
By studying microglial Orai1 knockout mouse models with sciatic nerve injury, they found that the loss of Orai1 mitigated pain hypersensitivity in male mice, but not in female mice. Male mice also had reduced induction of inflammatory cytokines — signaling molecules secreted from immune cells in response to injury — in spinal cord tissue but was not observed in female mice.
Next, both male and female mice were given a small-molecule Orai1 inhibitor compound called CM4620 and found that the compound mitigated neuropathic pain in male mice but had had no effect in the female mice.
“This study points to striking sex differences in the underlying mechanisms mediating pain hypersensitivity between males and females. We are now working on identifying whether Orai1 signaling can be manipulated in other immune cells to provide pain relief in female mice,” Prakriya said.
In addition to understanding how sex impacts the manifestation of pain and behavior, other Feinberg investigators have delved into how sex-based differences influence stress response which translates to the development of mental health disorders, particularly major depressive disorder (MDD).

Recent work led by Talia Lerner, PhD, assistant professor of Neuroscience and of Psychiatry and Behavioral Sciences, discovered how novel sex-specific mechanisms control how stress hormones impact dopamine transmission and motivation.
By studying changes in brains of mice given the stress hormone corticosterone (the rodent equivalent of cortisol), Lerner’s team discovered that chronic dysregulation of corticosterone impaired dopamine transmission in the dorsomedial striatum (DMS), a region of the brain that mediates decision-making and reward-seeking behaviors.
In male mice, corticosterone dysregulation reduced the function of the dopamine transporter in the DMS. In female mice, corticosterone dysregulation decreased the amount of dopamine in the DMS altogether.
The findings, published in the journal Neuropsychopharmacology, underscores that the design of new therapeutic strategies for MDD and other mental health disorders need to account for sex, gender and hormone status.
“We must continue to probe for sex differences at the molecular level if we are to appropriately translate preclinical discoveries into medicines that act at the molecular level,” Lerner said.






