
Investigators have discovered new mechanisms underlying the diversity of characteristics within a single tumor, as well as treatment resistance, in the most common type of primary central nervous system tumor, according to a recent study published in Nature Genetics.
The study, led by David R. Raleigh, MD, PhD, the Robert and Ruth Halperin Endowed Chair in Meningioma Research at the University of California San Fransisco, along with contributing author Amy B. Heimberger, MD, PhD, the Jean Malnati Miller Professor of Brain Tumor Research and vice chair for Research in the Department of Neurological Surgery, challenges current classification criteria for meningiomas and emphasizes the need for more personalized treatment strategies based on an individual tumor’s characteristics.
“Just in 2021, the World Health Organization revised grading criteria for meningioma to incorporate a modest but clinically significant number of molecular features into how these tumors are classified. But what we showed is that even though those tumors are all currently lumped together into the same grade, there’s dramatically different gene expression, biochemical and cellular programs underlying intratumoral heterogeneity in these clinically aggressive tumors,” Raleigh said.
Meningiomas account for approximately 40 percent of all primary central nervous system tumors. The tumor grows from meninges, tissue membranes that surround and protect the brain and spinal cord. While not a brain tumor, meningiomas can press on the brain and surrounding nerves and blood vessels.
In most cases, meningiomas are benign and can be removed with surgery. However, some meningiomas are aggressive. The standard of treatment for malignant, high-grade meningiomas is surgery followed by additional therapies, including radiation, molecular therapy, chemotherapy, or immunotherapy in the setting of clinical trials. Despite recent advancements in treatment approaches, however, the average five-year survival rate continues to be low, according to the National Cancer Institute, especially for tumors that are commonly considered “benign.”
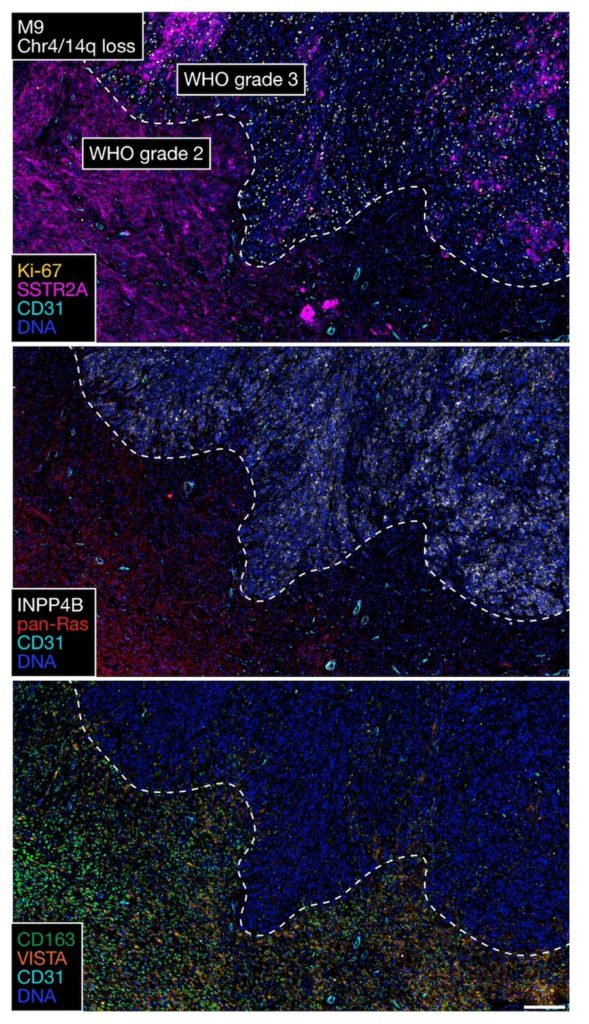
High-grade meningiomas are heterogeneous and, therefore, highly treatment-resistant, but the underlying mechanisms of the evolution and heterogeneity of these aggressive tumors have remained poorly understood.
“We know that intratumoral heterogeneity is a driver of resistance to cancer therapies, and so we hypothesized in this study that intratumoral heterogeneity at the level of single cells, spatial transcriptomes and regional protein expression programs might contribute to some of the more recalcitrant clinical behavior that we see,” Raleigh said.
In the current study, the investigators used multiple spatial approaches, including spatial transcriptomics and proteomics, to analyze 16 high-grade meningioma tumor patient samples to understand the genomic, biochemical and cellular drivers of intratumoral heterogeneity. The samples were then validated with a larger sample size consisting of over 500 meningiomas.
“Part of this heterogeneity has been the idea of one cell is different than another cell, but people really don’t understand that there’s almost neighborhoods and environments within that tumor that are distinctly unique and have distinctive underlying genetic and molecular drivers that are influencing that microenvironment,” said Heimberger, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
Using these approaches, the investigators uncovered dramatically different genomic, biochemical and cellular mechanisms underlying intratumoral heterogeneity in high-grade meningiomas that are otherwise grouped together by current meningioma classification criteria.
“What that tells us is that if we are going to develop new ways to treat these tumors, it probably can’t be a one-size-fits-all approach,” Raleigh said. “I think there needs to be molecular individualization.”
Next, to understand how the intratumoral heterogeneity evolves, the investigators used additional spatial approaches to study matched pairs of primary and recurrent meningioma tumors. They discovered that the recurrent tumors demonstrated completely different genomic, cellular and biochemical characteristics than the primary tumors, suggesting that therapeutic strategies need to prioritize and target how the tumor evolves, according to the authors.
“There is a real clinical need to understand, besides taking the tumors out with surgery, which ones are going respond to radiation? Do you have an applicable targeted therapeutic strategy? If so, which ones and is it going to sufficiently cover the cancer cells, and is there an immunotherapy strategy that could be employed?” Heimberger said.
Finally, to translate their findings to preclinical models, the investigators used epigenetic editing and lineage tracing approaches in human meningioma co-culture models to identify combinations of FDA-approved molecular therapies that target intratumor heterogeneity and inhibit meningioma growth.
The findings underscore the importance of prioritizing intratumor heterogeneity in meningioma classification criteria and underscore the need for more accurate preclinical models for high-grade meningioma clinical trials, according to the authors.
“Cancer classification, and cancer care more broadly, is evolving and I think figuring out the ways in which we can evolve how we classify tumors to optimize outcomes for patients is really important,” Raleigh said.
Co-authors include Hinda Nejem, PhD, a postdoctoral fellow in the Heimberger laboratory, Stephen Magill, MD, PhD, assistant professor of Neurological Surgery; and Craig Horbinski, MD, PhD, director of Neuropathology in the Department of Pathology. Magill and Horbinski are also members of the Lurie Cancer Center.
This study was supported by National Institute of Health grants T32 CA15102, P50 CA097257, P50 CA097257 777, F30 CA246808, T32 GM007618, P50 CA097257, F32 CA213944, R01 780 CA120813, R01 NS120547, P50 CA221747, R01 NS117104, R01 NS118039, P50 781 CA221747, R01 CA262311, P50 CA097257; the UCSF Wolfe Meningioma Program Project 778, 779 and 782; the Northwestern University Malnati Brain Tumor Institute; and the Trenchard Family Charitable Fund.






