Northwestern Medicine investigators led by Amy Heimberger, MD, PhD, the Jean Malnati Miller Professor of Brain Tumor Research and vice chair for Research in the Department of Neurological Surgery, have discovered a new mechanism in which cancer-associated fibroblasts mediate immune suppression in glioma tumors.
The findings, published in the Journal of Clinical Investigation, may also inform future immunotherapy strategies for patients who don’t respond to standard treatment options.
In solid tumors, cancer-associated fibroblasts (CAFs) — cells that form connective tissue — contribute to tumor formation and growth. CAFs have been known to reside in many solid tumor cancers and form a barrier around cancer cells to prevent them from being detected by T-cells.
Recent work has shown that CAFs are also present in glioblastoma, the most common and most aggressive primary brain cancer. This discovery prompted Heimberger’s team to investigate whether CAFs are a function of glioma grade or aggressiveness.
In collaboration with Michael DeCuypere, MD, PhD, assistant professor of Neurological Surgery in the Division of Pediatric Neurosurgery, the investigators used RNA sequencing to measure CAF frequency in low- and high-grade pediatric gliomas.
In high-grade gliomas, the investigators identified a higher frequency of a subtype of CAFs — immune modulatory cancer-associated fibroblasts.
“This study represents a unique look into the tumor microenvironment of pediatric high-grade gliomas, a previously understudied entity, and may open new avenues for immune-mediated therapy,” DeCuypere said.
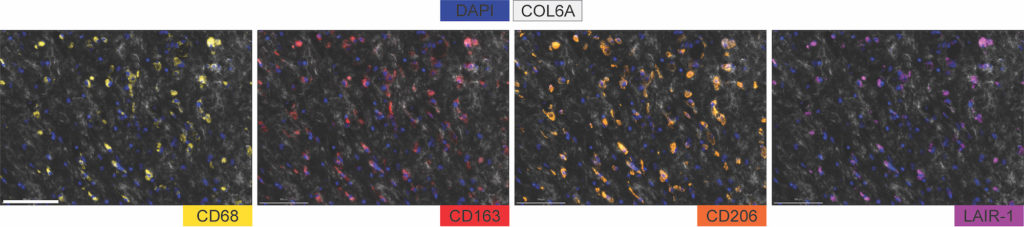
Multiplex staining revealed that these high-frequency CAFS were embedded in and produced collagen, which promotes tumor growth by triggering immunosuppressive mechanisms.
“These cancer-associated fibroblasts produce and are embedded in collagen, and this collagen contributes to the stiffness of the tumor,” Heimberger said.
Next, by analyzing single-cell RNA sequencing data from both pediatric low and high-grade gliomas, the investigators discovered that myeloid cells express an established immunosuppressive inhibitor, the LAIR1 protein, which is a key receptor for collagen. This receptor activates an immune inhibitory signal in myeloid cells (blood cells derived from bone marrow), which are frequent in gliomas, causing the myeloid cells to become unresponsive.

According to Heimberger, the findings could inform a new immunotherapy strategy for patients who don’t respond to traditional immune checkpoint inhibitors.
“Since the expression of PD-1 and PD-L1 in gliomas is very low and since these tumors do not typically respond to these types of immunotherapy, we have a new target that we can therapeutically consider,” Heimberger said.
Shashwat Tripathi, a student in the Medical Scientist Training Program (MSTP), and Hinda Najem, MD, MS, a postdoctoral fellow in the Heimberger laboratory, were co-lead authors of the study.
Co-authors include Jason Miska, PhD, assistant professor of Neurological Surgery, Matthew Tate, MD, PhD, associate professor of Neurological Surgery and in the Ken and Ruth Davee Department of Neurology; C David James, PhD, professor emeritus of Neurological Surgery, Craig M Horbinski, MD, PhD, director of Neuropathology in the Department of Pathology; Nitin Wadhwani, MD, assistant professor of Pathology in the Department of Pediatric Pathology; Maciej Lesniak, MD, the chair and Michael J. Marchese Professor of Neurosurgery; and Sandi Lam, ’98 MD, vice chair for pediatric neurological surgery in the Department of Neurological Surgery.
Heimberger, Miska, Tate, James, Horbinski and Lesniak are members of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
This research was supported by National Institute of Health grants CA120813, NS120547, CA221747 and CA060553, institutional funding from the Lou and Jean Malnati Brain Tumor Institute of the Lurie Comprehensive Cancer Center, and gifts from the Mosky family and the Stephen Coffman Trust.
Learn more about this work in a recent video from The Journal of Clinical Investigation.