This story is published in the August 2023 issue of the Breakthroughs newsletter.
Today, more than 50 million adults in the U.S. suffer from chronic pain — pain lasting longer than three months — and is the most common reason why people seek medical attention, according to the Centers for Disease Control and Prevention (CDC). For some, the cause of their chronic pain can be pinpointed to an injury site or chronic illness; for others, the causes are elusive and virtually untreatable.
The limited number of therapies for chronic pain is due to major gaps in the understanding of the underlying mechanisms of pain itself. As a result, there are no scientifically validated treatments available for chronic pain. However, recent breakthroughs made by investigators at Northwestern’s Center for Translational Pain Research are moving the field forward and suggest that the brain’s emotional circuity plays a causal role in chronic pain.

“It is a step away from treating the body part that’s injured into treating the whole organism and the response of the organism to that injury and how the organism is in fact reorganizing their brain circuits, their emotional interpretation of that injury. All of that becomes a critical part of what is the pain stage that they’re living in, so that gives us a whole new window where we can begin to look at components of those circuits and see if we can find new targets to treat pain,” said Apkar Apkarian, PhD, director of the Center for Translational Pain Research and a professor of Neuroscience, of Anesthesiology and of Physical Medicine and Rehabilitation.
The Center for Translational Pain Research was established in 2019 to expand collaborative, cutting-edge research that enhances the understanding of these mechanisms with the ultimate goal of leading to the development of novel non-opioid treatments managing and ameliorating acute pain and chronic pain conditions.
Since 1999, the number of opioid overdose deaths in the U.S. have quintupled; In 2020, opioids caused nearly 75 percent of all drug overdose deaths. In recent years, the CDC has ramped up its efforts encouraging healthcare providers to implement tapering — gradually decreasing a patient’s dosage — when caring for patients with opioid addiction. However, a recent study found this approach actually increased a patient’s risk of withdrawal and death.
With these findings top-of-mind, and this year marking the center’s five-year anniversary, Apkarian said the center’s commitment to having an impact on the opioid epidemic is stronger than ever.
“Our goals for the next five years are to try to understand the adaptations of the brain circuitry, both in humans and in animal models doing tapering, and to see if we can identify targets within those adaptations that we can then use to develop novel treatments for both chronic pain and also for dependence on opiates,” Apkarian said.
Collaboration at its Core
To accelerate bench-to-bedside research, the center partners with the Department of Anesthesiology, a symbiotic relationship that is fundamental for integrating basic science into clinical care and accelerating the development of novel therapies.
“We want to engage the clinicians within the Anesthesia Pain Center and bring them into our studies and collaborate,” Apkarian said.
For example, a recent study led by Apkarian and Paulo Branco, PhD, assistant professor of Anesthesiology in the Division of Pain Medicine, used machine learning to predict acute pain after mild traumatic brain injury (mTBI). By studying brain structural properties in brain white matter from mTBI patients, the investigators characterized specific neural networks underpinning early acute pain, their findings published in the journal Pain.
Translational pain research also permeates other departments across Feinberg and Northwestern. Investigators led by Talia Lerner, PhD, assistant professor of Neuroscience, discovered that dopamine signaling in the brain’s dorsomedial striatum promotes the development of compulsive behaviors in animal models, their findings published in Current Biology. These circuits are known to control the expression of compulsive behaviors commonly observed in obsessive-compulsive disorder (OCD) and substance misuse disorders and addiction.
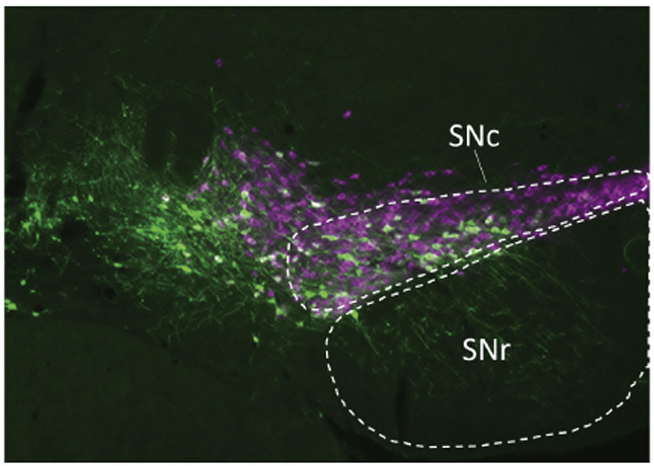
“Neurobiological variability is an interesting thing to take advantage of to understand how psychiatric illnesses might result from being on the tail ends of distributions, and looking what circuits control that variability in animal models will be really important for understanding human variability,” Lerner said.
Related work led by Rajeshwar Awatramani, PhD, associate professor in the Ken and Ruth Davee Department of Neurology, discovered that dopamine neurons have distinct projection patterns, expanding the understanding of the clinically important neurotransmitter system. The findings, published in Nature Neuroscience, could also improve the understanding of the cells’ role in different neuropsychiatric disorders, as well as chronic pain and drug addiction.
“Dopamine has been implicated in a spectrum of neuropsychiatric disorders including Parkinson’s disease, ADHD, depression, chronic pain and drug addiction. Our work opens the possibility that distinct dopamine subtypes may be involved in these diverse conditions,” Awatramani said.
Most recently, investigators led by John Rogers, PhD, the Louis Simpson and Kimberly Querrey Professor of Materials Science and Engineering, Biomedical Engineering and Neurological Surgery, developed a small, soft, flexible implant device that relieves pain on demand and without the use of drugs. The first-of-its-kind device, detailed in Science, could provide an alternative to opioids and other highly addictive medications.
“We have many different laboratories bringing in a lot of very rich technology that’s state-of-the-art, all of which is directed towards uncovering new potential targets for changing the whole landscape of both chronic pain and opiate abuse and dependence,” Apkarian said.






