
The protein FOXK2 promotes survival of cancer stem cells in ovarian cancer, according to a Northwestern Medicine study published in the Journal of Clinical Investigation.
Blocking this protein could reduce recurrence of cancer after initial treatment, according to Daniela Matei, MD, the Diana, Princess of Wales Professor of Cancer Research, chief of Reproductive Science in Medicine in the Department of Obstetrics and Gynecology and senior author of the study.
“If you use an inhibitor for this pathway, the cancer stem cells will die instead of regenerating a tumor,” said Matei, who is also a professor of Medicine in the Division of Hematology and Oncology.
When treated early enough, ovarian cancer usually follows a predictable course of remission after chemotherapy and subsequent relapse up to two years later. This long layoff may be caused by cancer stem cells (CSCs) — a small population of cells within tumors capable of self-renewal and differentiation. “Chemotherapy can eliminate most cells, but in some cancers, the stem cells are left behind, waiting until conditions are more favorable,” Matei said.
In the current study, Matei and her collaborators compared gene expression in ovarian CSCs and normal ovarian cancer cells, looking for genes with higher expression in CSCs. One gene with much higher expression in CSCs was one that coded for the protein FOXK2, a little-known transcription factor that has previously been studied in the context of metabolism.
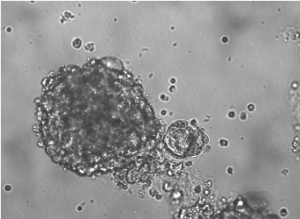
Investigators including lead author Yaqi Zhang, a student in the Driskill Graduate Program in Life Sciences (DGP), found FOXK2 directly regulated IREI-alpha, a key sensor that activates the unfolded protein response (UPR).
External stressors to cells — in the case of CSCs, chemotherapy or lack of oxygen from cells being clumped together — can cause misfolded proteins. The UPR ensures cells aren’t harmed by those malformed proteins, halting protein translation and degrading the misfolded proteins.
Blocking expression of FOXK2 in CSCs — and subsequently reducing activation of the UPR — inhibited growth of CSCs, reducing their so-called “stemness,” making them more like normal cells.
“We demonstrated this pathway is very important for stemness,” Zhang said.
The findings represent one possible method to target CSCs in cancer, Matei said. While current inhibitors are crude and have off-target effects, more sophisticated inhibitors could be used after chemotherapy to reduce recurrence of cancer.
“We are very interested in new inhibitors for this pathway,” Matei said.
Matei is a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
This study was supported by U.S. Department of Veterans Affairs grant BX000792-09A2 and National Cancer Institute grant U54 CA268084-02.






