Glioblastoma is one of the deadliest cancers known to man. While the advent of immunotherapy and other cutting-edge treatments have prolonged life for people afflicted with other types of cancer, the prognosis for glioblastoma has remained relatively constant: just 18 months.
That year-and-a-half can be brutal: bombarding the brain with radiation in an attempt to crush the cancer into submission, often with little success. Glioblastoma is notoriously resistant to therapy, quickly adapting and roaring back with deadly results.

“It’s not an exaggeration to say that nearly every glioblastoma patient will, unfortunately, succumb to the cancer. It is, in nearly all cases, incurable,” said C. David James, PhD, professor emeritus of Neurological Surgery.
The lethality of glioblastoma and the paucity of effective treatments is what spurred Maciej Lesniak, MD, chair and Michael J. Marchese Professor of Neurosurgery, along with James, to apply for the Specialized Program of Research Excellence (SPORE) grant from the National Cancer Institute, to be awarded to the Robert H. Lurie Comprehensive Cancer of Northwestern University. They didn’t do this alone: The 2017 arrival of renowned neuro-oncologist Roger Stupp, MD, the Paul C. Bucy Professor of Neurological Surgery and chief of Neuro-Oncology in the Department of Neurology, bolstered the glioblastoma expertise at Northwestern, and his continued leadership has been a tremendous boon to the program, Lesniak said.
Northwestern’s Brain Tumor SPORE — part of the Lurie Cancer Center — is now three years old, and the bench to bedside process is producing results. Under the leadership of Lesniak and James, the SPORE has made advances in understanding the genetic basis of the disease and developed potential therapies that reduce treatment resistance and clinical trials using immunotherapies. The SPORE philosophy of collaboration and team science under one roof is alive and well.
Genetics of glioblastoma
Since The Cancer Genome Atlas (TCGA) published its landmark 2008 analysis of the genetics of glioblastoma, scientists such as Alexander Stegh, PhD, associate professor in the Ken and Ruth Davee Department of Neurology Division of Neuro-Oncology, have used that roadmap to guide their research.
“The TCGA gave us this ‘periodic table’ of genes that are deregulated in glioblastoma,” said Stegh, who is also an associate professor of Medicine in the Division of Hematology and Oncology.

While some cancers have oncogene activations that are relatively simple to single out, there’s an emerging understanding that glioblastoma is caused by variants of many genes. This is why previous attempts at therapies targeting single genes failed, such as those targeting alterations of the EGFR gene, and why Stegh focuses on genetic deregulation that contributes to therapy resistance.
“Rather than going in there with the very ambitious goal of identifying multiple genes and dialing down their expression levels, we take a slightly different approach: How can we specifically downregulate genes that cause therapy resistance, as an adjuvant therapeutic approach,” Stegh said.
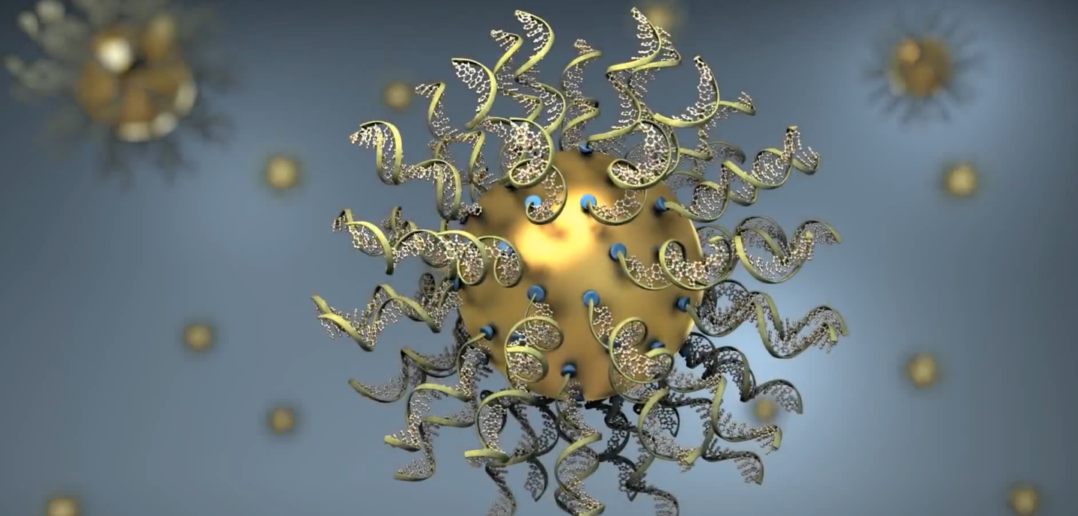
Stegh has published several papers identifying important genes implicated in glioblastoma therapy resistance, but one gene, called Bcl2L12, was found to be especially amenable to therapeutic delivery.
Combining his genetic expertise with the nanotechnology expertise of Chad Mirkin, PhD, professor of Medicine in the Division of Hematology and Oncology; and the clinical trial expertise of Priya Kumthekar, MD, ’08 ’11 ’12 GME, associate professor of Neurology in the Division of Neuro-oncology, the investigators designed a spherical nucleic acid drug that crossed the blood-brain barrier and primed tumor cells for death.
The trial, published in Science Translational Medicine, was the first of its kind to show that a nano-therapeutic crossed the blood-brain barrier and into brain tumor cells in patients.
“This unique 3D design has the ability to infiltrate tumor cells to correct the genes inside and make them susceptible for therapy-induced killing,” Stegh said.
Bcl2L12 was initially identified as a treatment target by Stegh in 2007. “To go from identifying this gene during my postdoctoral work, to get to the point of actually targeting it and establishing proof-of-concept in patients, it’s very gratifying,” Stegh said. “We are looking forward to building on this success.”
Breaking through
A recurrent obstacle in glioblastoma treatment is the blood-brain barrier. Efforts to develop treatments beyond simple chemotherapy are often stymied by the selective permeability of the barrier, but projects in the SPORE are using emerging technologies to break through. Beyond the project using SNA’s, a group of investigators led by Lesniak used stem cell “shuttles” to deliver immunotherapy directly to the tumor site.
Neural stem cells have an affinity for the brain, often traveling to areas of injury. Taking advantage of this travel pattern, investigators modified neural stem cells to produce an oncolytic virus, which targets cancer cells and jump-starts the body’s immune response.
The phase I clinical trial, published in The Lancet Oncology, found that this approach was safe and tolerable for patients, and even showed signs that the treatment may improve progression-free and overall survival.
“This is the first-in-human clinical trial to test the neural stem cell delivery of an engineered oncolytic adenovirus,” Lesniak said.
Planning for the future

This emphasis on results — or clinical trials testing therapies — is what unites all members of the Brain Tumor SPORE. Kumthekar, who has a hand in nearly all clinical trials coming out of the SPORE, chalks up their success to two things: planning and people.
“When we are testing drugs in the pre-clinical phase, we are planning the early clinical phase I. When we are in phase I, we are planning phases II and III,” Kumthekar said. “We are always planning the next phase with the goal to get drugs that work to patients as fast as possible.”
Further, the wealth of bright minds within the Lurie Cancer Center have made collaboration seamless and stimulating for participating faculty. From her work with Stegh and Mirkin, to pre-clinical work with Atique Ahmed, PhD, associate professor of Neurological Surgery, the greatest resource of the Brain Tumor SPORE has been its people, Kumthekar said.
One collaborative project between Kumthekar, Ahmed and Stupp, found that a drug currently used to prevent organ rejection in transplant patients could also reduce chemotherapy resistance in glioblastoma. Published in Brain, investigators found this drug blocks one molecular synthesis pathway used by cancer cells being treated with radiation therapy; when unable to create molecules essential for DNA synthesis, the cancerous cells are more likely to succumb to the therapy and die.
Back-and-forth collaboration between Kumthekar and Ahmed — bringing clinical trial and laboratory expertise together — is part of why this drug was selected by the Alliance for Clinical Trials in Oncology, part of the National Clinical Trials Network (NCTN). As a lead academic participating site, Lurie Cancer Center provides scientific leadership in the development and conduct of clinical research within the NCTN, and planning for the phase I trial at Northwestern is already in full swing. A potential phase III trial could be at several alliance network locations around the U.S., according to Kumthekar.
“The field is very interested in drug repurposing right now, and this helps us speed availability of drugs to patients,” Kumthekar said.
The end goal of patient care is what unites all members of the SPORE — from laboratory-based scientists to clinical trial experts — and as these therapies march forward through the lengthy process of clinical trial evaluation, some scientists are hopeful that better treatments are just around the corner.
“Over the last ten to fifteen years, our body of knowledge about the molecular characteristics of glioblastoma has increased tremendously,” James said. “As we take the information generated by dozens, if not hundreds of labs and analyze individual patient tumors to determine characteristics that can be targeted with specific therapies, I think we will begin to see more rapid progress in effective treatment of this cancer.”
Lesniak, James, Stupp, Stegh, Mirkin, Kumthekar and Ahmed are members of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University and part of the Lou and Jean Malnati Brain Tumor Institute at the Lurie Cancer Center. Lesniak is director of neuro-oncology at the Lurie Cancer Center. Lesniak and James are principal investigators of Lurie Cancer Center’s Brain Tumor SPORE.