Boosting function of natural killer cells with magnetic nanoparticles could make cancer immunotherapy more efficient, according to a Northwestern Medicine study published in ACS Nano.
This method could unlock the potential to use natural killer (NK) cells on a variety of solid tumors, according to Dong-Hyun Kim, PhD, associate professor of Radiology in the Division of Basic and Translational Radiology Research and senior author of the study.
“People have had trouble applying NK cells to solid tumors,” said Kim, who is also director of Biomaterials for Image Guided Medicine (BIGMed) laboratory and a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. “If we can provide an easy path to modulate NK cells, perhaps this can become a useful therapy.”
Most cell-based immunotherapies target T-cells, part of the body’s adaptive immune system. However, these chimeric antigen receptor (CAR) T-cell therapies come with a high price tag, long incubation period and strong side effects.
On the other hand, NK cells are part of the body’s innate immune system and quicker to respond to anything foreign. Many scientists have explored the possibility of NK cell immunotherapy, according to Kim, but that too has barriers.
“It’s pretty hard for these cells to penetrate inside the tumors which have thick barrier tissues,” Kim said.
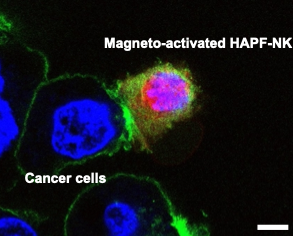
Methods to boost NK cell function using cytokines have largely fallen flat and are subject to some of the same problems as seen in CAR T-cell therapy — high cost and lengthy manufacturing time. However, Kim’s previous work with nanoparticles inspired a different approach.
Kim and his collaborators designed a magnetic nanocomplex that binds with NK cells and when activated with an alternating magnetic field, exerts force on the exterior of the cell, promoting secretion of cytotoxic compounds. Testing this nanocomplex in animal models of hepatocellular carcinoma, the investigators found that magnetic activation increased the cancer-killing ability of NK cells when injected into solid tumors.
Further, these nanoparticles are easily visualized on magnetic resonance imaging, allowing for precise monitoring of NK cell distribution during and after injection.
“This creates a stronger NK cell, and can hopefully enhance the efficacy of the treatment,” Kim said.
Taehoon Sim, PhD, postdoctoral fellow in the Kim laboratory, was lead author of the study.
This work was mainly supported by grants R01CA218659 and R01EB026207 from the National Cancer Institute and National Institute of Biomedical Imaging and Bioengineering. This work was also supported by the Center for Translational Imaging and the Mouse Histology and Phenotyping Laboratory at Northwestern University.