
A team of Northwestern Medicine investigators has discovered a set of intracellular mechanisms that support the polarized function of the skin’s outermost layer, the epidermis, according to findings published in Current Biology.
The study, led by Kathleen Green, PhD, the Joseph L. Mayberry, Sr., Professor of Pathology and Toxicology and associate director for Basic Science Research at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, has the potential to improve understanding of how these key functional drivers contribute to the development of multiple skin diseases.
Epithelial tissue is protective tissue that lines the outside layer of the skin and internal organs. A unique characteristic of this tissue is its polarization, with the upper and lower layers of the epithelial cells performing different functions to regulate the passage of molecules and protect the body from foreign substances and pathogens.
In the case of the epidermis, it is polarized across the entire tissue over multiple cell layers, known as apicobasal polarity — the outermost layer, or apical cell layer, acts a protective barrier whereas the innermost layer, or basal cell layer, supports cell regeneration. However, little is known about how this polarity is created and sustained, according to the authors.
“We’re hypothesizing that it’s different mechanical properties in the epithelial cell that are driving layer-specific functions,” said Joshua Broussard, PhD, research assistant professor of Pathology, of Dermatology and lead author of the study.

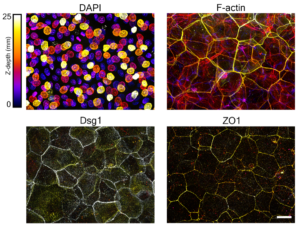
Using genetic interference, microscopy and biochemical techniques to analyze human reconstructed epidermis in vitro, the investigators identified adhesive protein complexes called desmosomes and cytoskeletal support structures called intermediate filaments as central regulators of mechanical polarization in the epidermis.
Furthermore, the investigators found that basal layer mechanics and dynamic movement of cells into the suprabasal layers could be reduced by uncoupling desmosomes and intermediate filaments, a process that involves inserting a form of the protein desmoplakin which binds to desmosomal proteins but lacks the ability to bind to intermediate filaments. The team also found that removing the protein desmoglein-1, a cell-cell adhesion molecule anchored to the intermediate filament cytoskeleton, also produced similar results. Further, disconnecting intermediate filaments from the desmosome hindered the establishment of barrier function in the outer layers of the epidermis.
“So we’re seeing that if we alter the desmosome-intermediate filament connection, that it’s altering this tension stiffness gradient in the epidermis, and that has an impact on the barrier function,” Broussard said.
According to the authors, the findings may be used in studying different types of mechanical forces in the epidermis on specific body parts that lead to prominent skin conditions such as psoriasis or eczema, as well as to improve treatment regimens for these diseases.
Broussard and Green are members of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
This work was supported by National Institutes of Health grants R01 AR041836, R37 AR043380, R01 CA228196, F31 AR076188, K01 AR075087 and T32 AR060710; the J.L. Mayberry Endowment; and the Chicago Biomedical Consortium with support from the Searle Funds at The Chicago Community Trust.






