
Northwestern Medicine investigators have discovered that inhibiting tumor-associated myeloid cells’ ability to produce specialized metabolites called polyamines may improve the effectiveness of immunotherapy and other treatments for glioblastoma, according to a study published in Science Advances.
Tumor-associated myeloid cells can make up as much as 50 percent of a tumor’s mass, promoting tumor growth and enhancing immune suppression. Within solid tumors are very harsh microenvironments, ones that are very hypoxic, acidic and low in nutrients, making it challenging for cells to survive — except for those myeloid cells.
“The big picture question we had is: why are these cells there, and how do they survive in a tumor?” said Jason Miska, PhD, research assistant professor of Neurological Surgery and lead author of the study.
For the current study, Miska and colleagues utilized mouse models of glioblastoma, as well as metabolomic and transcriptomic analysis to determine what differences exist within tumor-associated myeloid cells, as compared to other tumor-infiltrating cells, such as CD8+ T-cells. The team discovered that the arginine-ornithine-polyamine cellular pathway is upregulated in tumor-associated myeloid cells, but not in the T-cells.
Notably, this pathway is involved in using arginine — an amino acid used for the biosynthesis of proteins — to make polyamines, molecules that are essential for cell survival and differentiation.
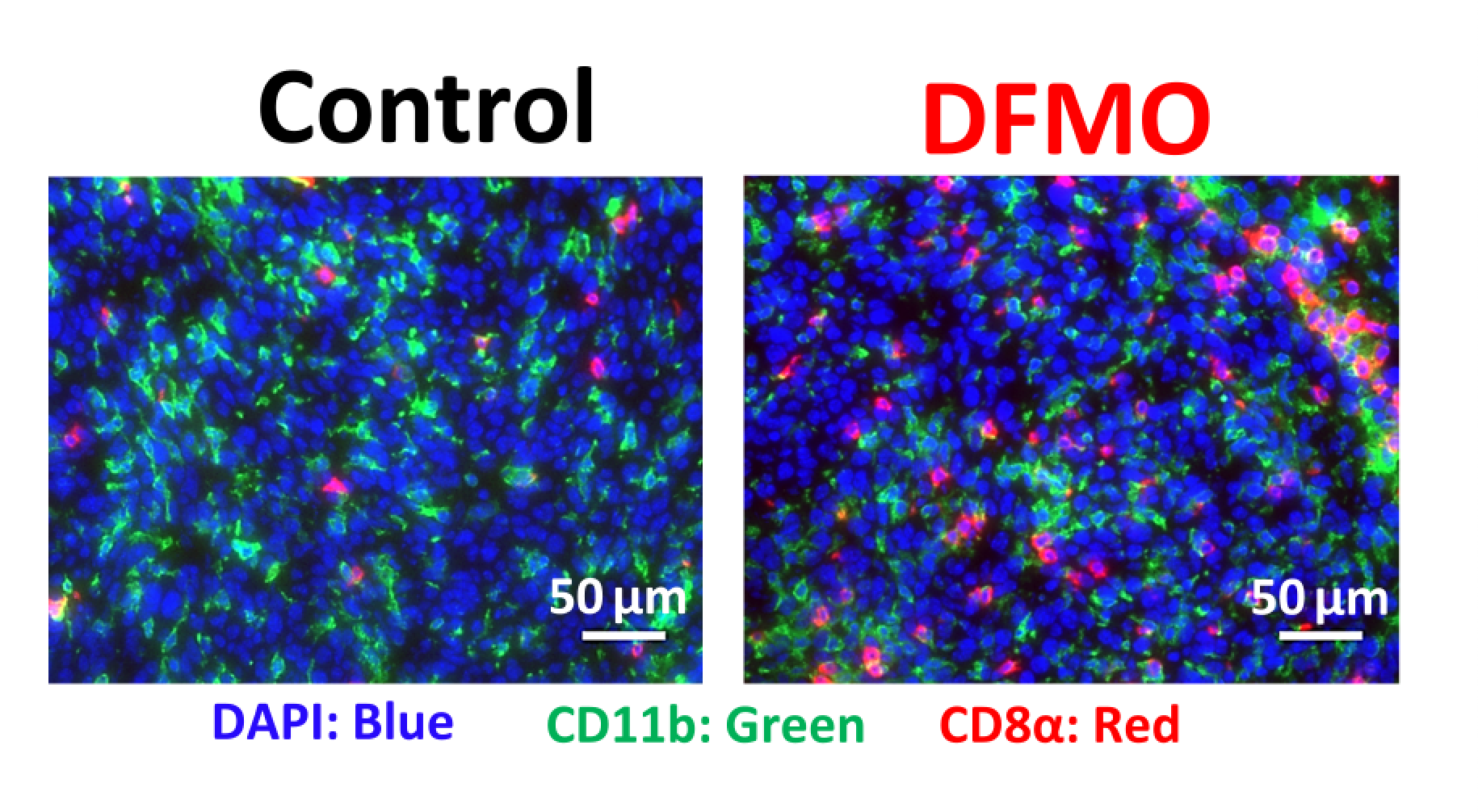
“Myeloid cells have an innate ability to make polyamines in the tumor to keep themselves protected from the acidity, and that protection allows them to function and thrive within the tumor,” Miska said.
The team then tested a polyamine inhibitor to block myeloid cell’s generation of new polyamines, which caused the myeloid cells to die and helped the tumor microenvironment become more permissive for immunotherapy.
Overall, this targeted therapeutic approach could be effective treating glioblastoma, which is notorious for being immunosuppressive, and may be able to be used in combination with other therapies, according to the authors.
Maciej Lesniak, MD, the chair and Michael J. Marchese Professor of Neurosurgery, was senior author of the study. Co-authors included Catalina Lee Chang, PhD, research assistant professor of Neurological Surgery, Peng Gao, PhD, research assistant professor of Medicine in the Division of Pulmonary and Critical Care, Peng Zhang, PhD, research assistant professor of Neurological Surgery, Craig Horbinski, MD, PhD, director of Neuropathology in the Department of Pathology, and Navdeep Chandel, PhD, the David W. Cugell, MD, Professor of Medicine in the Division of Pulmonary and Critical Care.
Miska, Lesniak, Lee Chang, Gao, Zhang, Horbinski and Chandel are members of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
This work was supported by the Northwestern University RHLCCC Flow Cytometry Facility and a Cancer Center Support Grant CA060553, the National Institute of Neurological Disorders and Stroke grants 1R01NS115955-01 and R01NS093903, the National Cancer Institute Outstanding Investigator Award R35CA197532, and a sub-award P50CA221747 from the Specialized Program of Research Excellence (SPORE) grant from the National Institutes of Health for Translational Approaches to Brain Cancer.






