
A protein known mostly for its role in the brain may be a key player in the cardiovascular system as well, according to a Northwestern Medicine study published in Nature.
These findings show that in mice, the Reelin protein promotes cardiac regeneration and repair by reducing cell death after heart injury. Importantly, these findings highlight a new strategy to improve cardiac function after heart attack, according to Guillermo Oliver, PhD, the Thomas D. Spies Professor of Lymphatic Metabolism and senior author of the study.
“Improving proliferation of mammalian cardiomyocytes has so far proven very difficult,” said Oliver, who is also a professor of Medicine in the Division of Nephrology and Hypertension and director of the Center for Vascular and Developmental Biology at the Feinberg Cardiovascular and Renal Research Institute. “Based on this study, reducing cardiomyocyte death could be an alternative approach to improve cardiomyocyte proliferation, and Reelin seems to be very efficient in performing such a job. Furthermore, the use of both strategies simultaneously could provide a synergistic effect.”
Cardiomyocytes, the cells that make up heart muscle, are the most prevalent cell type in the heart. These oblong-shaped cells rhythmically contract and relax, generating force that pumps blood through the heart and out to the rest of the body.
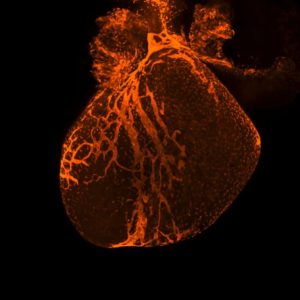
In cardiovascular conditions such as heart disease and heart attack, blood circulates more slowly through the heart or stops altogether. This deprives these cells of oxygen and other essential nutrients, often killing large swathes of cardiomyocytes and leaving scar tissue stretched across the heart.
In adult mammals, cardiomyocytes do not regenerate; Therefore, the heart has little ability to recover heart function after injury. This can leave patients with permanent cardiac function deficits for the rest of their lives, according to Oliver.
Heart disease is a leading cause of mortality in the developed world and many scientists are investigating how to stimulate cardiomyocyte proliferation as a tool to improve cardiac repair, Oliver said. While techniques like stem cells or transplanted cells have been explored in clinical trials, those efforts have proven difficult.
However, these findings from the Oliver laboratory suggest an alternative approach.
Oliver’s laboratory studies the lymphatic vasculature, the network of vessels that transport inflammatory cells all over the body. During the past decade, new functions of the lymphatic system have been uncovered, including roles in obesity, neurological disorders, glaucoma and cardiovascular disease.
In the current study, Xiaolei Liu, PhD, research assistant professor of Medicine in the Division of Nephrology and Hypertension and lead author of the study, started by analyzing mouse models lacking the lymphatic vasculature during embryonic development. Surprisingly, the investigators found that the heart was significantly smaller in these embryos, a consequence of severely reduced cardiomyocyte proliferation and increased cardiomyocyte cell death.
“This was the first indication that lymphatics are required for embryonic cardiomyocyte proliferation and heart growth,” Oliver said.
Using proteomic analysis, the scientists identified Reelin — a protein secreted from lymphatic cells — as a factor required for cardiomyocyte proliferation and heart growth. Next, they used another mouse model, this time without Reelin. They found that cardiac regeneration and function is impaired in young mice after cardiac injury.
“In mice, heart regeneration is possible until day seven after birth. Afterwards, that ability is gone as cardiomyocytes cannot proliferate any longer,” Oliver said. “Our results showed that without Reelin, cardiac regeneration and function after heart attack is severely impaired in these young pups.”
It appeared that Reelin was important for cardiac regeneration, so Liu took the study one step further: In normal adult mice, they induced heart attacks, then embedded recombinant Reelin protein into the heart. Weeks later, these mice experienced improved cardiac repair and function.
Critically, the investigators also found that Reelin was not boosting production of new cardiomyocytes — instead, it was preserving cardiomyocytes that might otherwise die.
“This was very exciting,” Liu said. “We were surprised this worked in adult mice, and it lead us down this whole different path.”
In the future, Oliver said he hopes to study this further, hopeful that it might produce an effective therapy for improving heart function after injury.
“I’m pretty confident this could be a valuable alternative approach to follow in various cardiovascular diseases. Many laboratories are trying to improve cardiac function by boosting proliferation, but we believe that reducing cardiomyocyte cell death could lead to possible paths to improve cardiac regeneration and cardio-protection,” Oliver said. “We believe that in the future, the use of this protein could hopefully improve cardiac function after injury in affected individuals.”
Oliver is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
This work was supported by National Institutes of Health grants RO1HL073402-16, T32 GM00806, HL63762, and NS093382; American Heart Association grants 18CDA34110356 and 5T32HL134633; a grant from the Spanish Ministry of Education, Culture and Sports; an EMBO Short-Term Fellowship; Leducq grant TNE-17CVD; an RD16/0011/0019 grant from the Spanish Ministry of 35 Science, Innovation, and Universities; grants VEKOP-2.3.2-16-2016-00002, NVKP_16-2016-1-0039 and Higher Education Institutional Excellence Program of the Ministry for Innovation and Technology in Hungary within the framework of the Molecular Biology thematic program of the Semmelweis University.






