
A recent Northwestern Medicine study published in the Journal of Experimental Medicine has revealed previously unknown details about calcium signaling that can contribute to inflammation.
These findings may help scientists better understand how to regulate inflammation, in an attempt to create new therapies for conditions such as inflammatory bowel disease and atherosclerosis, according to William A. Muller, MD, PhD, the Janardan K. Reddy, MD Professor of Pathology and senior author of the study.
“This paper shows for the first time how calcium signaling in endothelial cells, which is critical for inflammation under all conditions, is restricted in time and space,” said Muller, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. “In the future, we want to explore how targeting endothelial-cell specific machinery can limit inflammation, but keep the body’s ability to respond to new inflammatory stimulus.”
Prarthana Dalal, a fourth year medical student in the Medical Scientist Training Program (MSTP) was first author of the study, and David Sullivan, PhD, research assistant professor of Pathology, was a co-author.
White blood cells, or leukocytes, are the body’s foot soldiers: patrolling the bloodstream, waiting for chemical alarms that trigger a coordinated charge to sites under attack. To do this, they must cross endothelial cells that line the blood vessels of the body.
Getting from the bloodstream into tissue requires the help of a membrane complex called the endothelial lateral border recycling compartment (LBRC), which surrounds the leukocytes and shepherds them across cell junctions and into tissue on the other side. Previous work in the Muller laboratory demonstrated that LBRC movement was triggered by calcium signaling, but the specifics of the mechanism were not clear, Muller said.
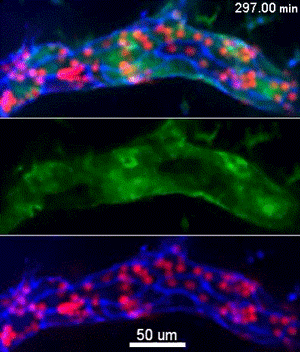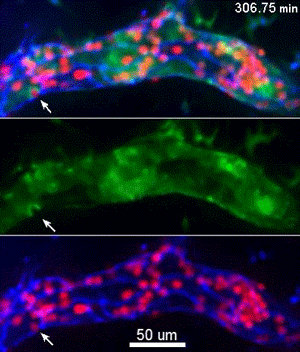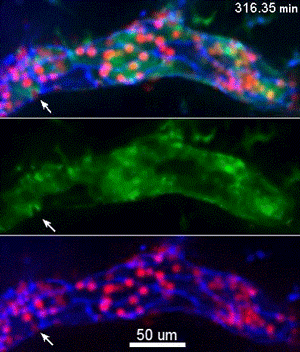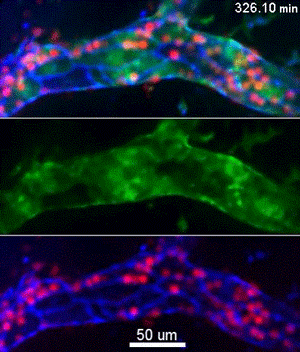
To investigate, Muller and his collaborators bred mice whose endothelial cells glowed bright green in proportion to the concentration of free calcium ions in their cytoplasm. Then, the scientists gave these mice a bone marrow transplant from mice whose neutrophils, a specific type of white blood cell, glowed bright red. This allowed the investigators to track the inflammatory response and blood cell migration by microscopy as it unfolded in real time.
They discovered a self-contained process where molecules on the leukocyte engage molecules in the endothelial cell wall, leading to an increase in calcium flow that begins just as leukocytes start transmigration and ends just before they complete it.
“Calcium signaling happens all over the cell, so all parts of this signaling process are localized to the endothelial cell borders as to only trigger transmigration and not a whole other range of calcium signals,” Muller said.
These findings provide a better framework for modulating inflammation, which could be used to treat diseases of inflammation such as inflammatory bowel disease or atherosclerosis, according to Muller.
Current therapies block leukocytes throughout the body, which can have serious side effects. Instead, Muller envisions a drug that selectively targets the endothelial cells to decrease the amount of white blood cells getting through the endothelium, in the hopes of controlling excess inflammation while maintaining leukocyte function.
“Blocking endothelial-specific molecules that regulate transmigration is never 100 percent effective — that’s actually good news,” Muller said.
Using the example of Crohn’s disease, Muller said he hopes an endothelial migration-blocking drug could reduce the number of leukocytes traveling into the gut by 80 or 90 percent — enough to reduce symptoms locally, but not interfere with the function of the remaining leukocytes.
“This might be enough to manage patients’ diseases while not interfering with the body’s ability to respond to new inflammatory challenges,” Muller said.
This work was supported by National Institutes of Health grants R01HL046849, R01HL064774, T32GM8152, F30HL134202, NIH R01HL108932 and R01HL145459, and an Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship.






