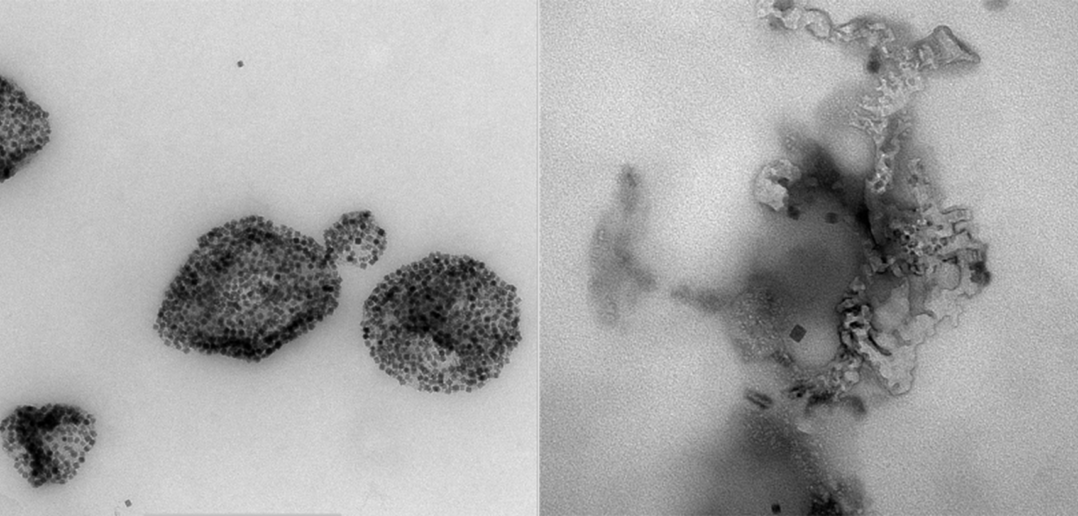
Iron nanoparticles in a polymer shell could be one day used to treat cancer, according to a Northwestern Medicine study published in Nature Communications.
This burgeoning treatment strategy kills cancer cells by overloading their iron metabolism, causing a buildup of toxic compounds within the cell. Now, Northwestern Medicine scientists have created a new shelled particle, with better results compared to early attempts.
“This shows there is a real potential for ferroptosis-based therapy,” said Dong-Hyun Kim, PhD, associate professor of Radiology in the Division of Basic and Translational Radiology Research and senior author of the study.

Traditional cancer treatments such as radiation-based therapies often target non-cancerous cells as well, causing significant and uncomfortable side effects. While immunotherapy is an expanding treatment approach that boosts a patient’s own anti-tumor abilities, its use is still limited to just a few types of cancer, as many tumors don’t respond to immunotherapy.
However, an early-stage treatment strategy is piquing the interests of scientists and clinicians: ferroptosis.
Ferroptosis is an alternate method of causing cell death. Rather than bombarding cells with radiation, or inducing the immune system to attack cancer cells, this strategy uses iron nanoparticles to jump-start the Fenton reaction, part of the normal process of iron metabolism in cells.
The reaction normally produces reactive oxygen species as a byproduct. But when additional iron is introduced into the cells, it runs hot and produces many more reactive oxygen species which can weaken or kill the cell.
In the current study, investigators created a more sophisticated particle to induce ferroptosis: a hybrid core-shell vesicle. The core is made of iron and ascorbic acid, which is a precursor for vitamin C and a necessary component of the Fenton reaction. This addition is intended to further boost the Fenton reaction, according to Kim.
The shell is made of a polymer and shields the core until an alternating magnetic field is applied — in this case, from a magnetic resonance imaging (MRI) machine. The field moves the small iron particles back and forth very rapidly, breaking the shell and releasing the core particles into the body.
Kim and his team found that this new shelled particle was more effective at destroying tumor cells, both in petri dishes and in mouse models of cancer when compared to older versions of the iron particles. Furthermore, activating these particles with an MRI allowed the investigators to determine when and where the particles attacked cells by selectively applying the magnetic field.
“This on-demand treatment release can be very important for decreasing side effects and for focusing the magnetic field on just the tumor region,” said Kim, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
The treatment also allowed T-cells to infiltrate into the tumors, demonstrating that ferroptosis could be combined with immunotherapy to selectively fight cancer, potentially with fewer side-effects than radiation therapy.
“Immunotherapy is popular, and maybe these two treatments could be a beneficial combination,” Kim said.
This work was mainly supported by grants R01CA218659 and R01EB026207 from the National Cancer Institute and the National Institute of Biomedical Imaging and Bioengineering. This work was also supported by the Center for Translational Imaging and the Mouse Histology and Phenotyping Laboratory at Northwestern University.






