A team of investigators have uncovered the cellular mechanisms of a specialized group of white blood cells that help promote the development of inflammatory diseases, according to a recent study published in the Proceedings of the National Academy of Sciences.
The cells, called neutrophils, are white blood cells that act as a first line of defense in the immune system. During infection, a process called NETosis occurs, in which neutrophils expel DNA that forms a net-like extracellular structure made up of histones and cytotoxic proteins to trap pathogens and promote blood clotting.
The problem, however, is that NETosis, which is dependent on an enzyme called PAD4, can also promote inflammation and cause additional damage to the body.
Furthermore, the cellular events that drive NETosis and the role of PAD4 in these events have up until now remained unclear, according to Robert Goldman, PhD, professor of Cell and Developmental Biology, of Medicine in the Division of Pulmonary and Critical Care, and co-author of the study.
Using various high-resolution microscopic imaging techniques, the authors characterized the timing of dynamic cellular events leading up to NETosis in live mouse and human neutrophils, labeled with fluorescent markers.
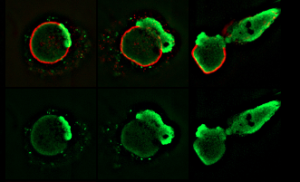
“In order to capture the dynamics of the process and to show that it actually proceeds in a series of steps, which turned out to be more steps than we thought and are very, very complicated, a lot of assays were recorded in vivo using time-lapse imaging,” said Goldman.
The cells were then stimulated with various reagents and a bacterial yeast species Candida albicans to induce the process of NETosis. Ultimately, they found that NETosis requires the activity of the PAD4 enzyme for DNA to be released from the nucleus and cell membrane in neutrophils.
Specifically, the stimulated cells exhibited an abrupt and rapid disassembly of various cytoskeletal structures within the cytoplasm, followed by the breakage of the nucleus. This sequence of intracellular mechanisms ultimately involved the rupturing of the plasma membrane and the discharging of DNA into the extracellular environment.
“We’re very excited about this, because our findings illustrate a series of steps involved in the process and that there is a precise sequence of changes,” said Goldman, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
“Many potential therapeutic targets have been revealed in this process of NETosis. These targets have the potential to control NETosis in chronic inflammatory diseases” Goldman added.
This work was supported by National Institutes of Health grants R01 GM106023, P01 GM096971 and T32 CA08062160038140.