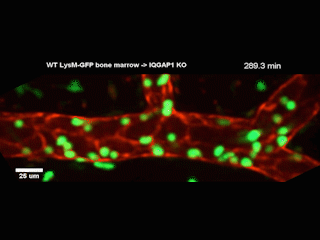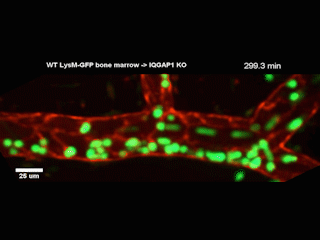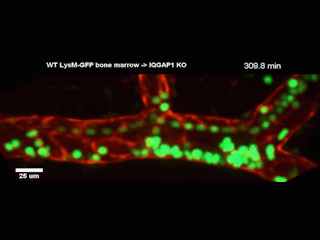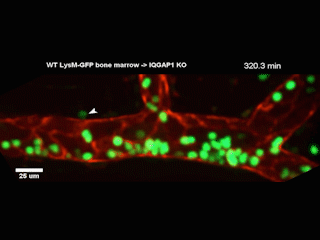
A Northwestern Medicine study has discovered new details of a mechanism that facilitates white blood cells traveling from blood vessels into tissue, according to findings published in the Journal of Experimental Medicine.
This process is crucial for the body’s inflammatory response, and the ability to modify that response could help treat a variety of conditions, according to William Muller, MD, PhD, professor of Pathology and senior author of the study.
“Inflammation is at the root of all pathology,” according to Muller, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. “Interrupting this pathway could be a good way to block unwarranted inflammation, and stimulating the pathway could be a good way to promote inflammation, such as when the body is healing a wound or getting rid of bacteria.”
White blood cells, or leukocytes, are the body’s foot soldiers: patrolling the bloodstream, waiting for chemical alarms that trigger a coordinated charge to sites under attack.
However, getting from the bloodstream into the tissue — known as diapedesis — requires the help of a complex called the endothelial lateral border recycling compartment (LBRC).

When white blood cells arrive at blood vessel walls, the LBRC latches onto them and surrounds them with a membrane, shepherding the white blood cells through the wall and into tissue on the other side. Previously, the precise mechanisms regulating this interaction were largely unknown, according to Muller.
In the current study, investigators examined a protein called IQGAP1, well-known for its role in regulating a wide range of cellular processes. They produced cell models wherein one of IQGAP1’s domains, or constituent parts, was disabled in each model. By observing how diapedesis was affected, the investigators determined that two of the domains were essential for diapedesis.
The first, the calponin homology domain, is responsible for localizing LBRC to the site along the endothelial junctions where the white blood cells are trying to cross, according to David Sullivan, PhD, research assistant professor of Pathology and lead author of the study.
“IQGAP1 can take signals from various pathways, and almost like a computer, give an output that’s meaningful,” Sullivan said. “In this case, the domain seems to be a vehicle that says, ‘Here, come meet the leukocyte.’”
The second influential domain was the IQ motif domain, which appears to direct calcium within the endothelial cell, according to Prarthana Dalal, a sixth-year student in the Medical Scientist Training Program (MSTP) and second author of the study.
“There’s an influx of calcium into the cells that helps during this trans-migration process,” Dalal said. “Once the calcium is inside, this domain helps it get where it needs to go.”
The discovery of this protein’s effects on diapedesis could have future therapeutic implications, the authors said. A wide variety of events can lead to inflammation, which can be harmful when there’s too much or too little involved, and all inflammation relies on this single mechanism.
If scientists could modify LBRC via the IQGAP1 protein, Muller said, it may represent a way to fine-tune inflammation without disturbing other processes in the body.
“You wouldn’t want to target IQGAP1 or calcium signaling all throughout the body because that would probably be incompatible with life,” Muller said. “Instead, if you could use IQGAP1 to dial in the level of inflammation you want, I could foresee using this at low levels for chronic inflammatory diseases like atherosclerosis. That would helpfully impair inflammation but not interfere with the body’s ability to fight off bacteria.”
This work was supported by National Institutes of Health (NIH) grants F32 AI084454, R01 HL046849, R21 HL102519, R37 HL064774, F30HL134202, T32GM8152, R01GM087575, an Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship, the Intramural Research Program of the NIH and National Institute of General Medical Sciences.





