Guillermo Oliver, PhD, the Thomas D. Spies Professor of Lymphatic Metabolism, recently published two studies related to the eye, one on retinal formation and the other on the genetics behind glaucoma.
Stem cells lead to better understanding of retinal development
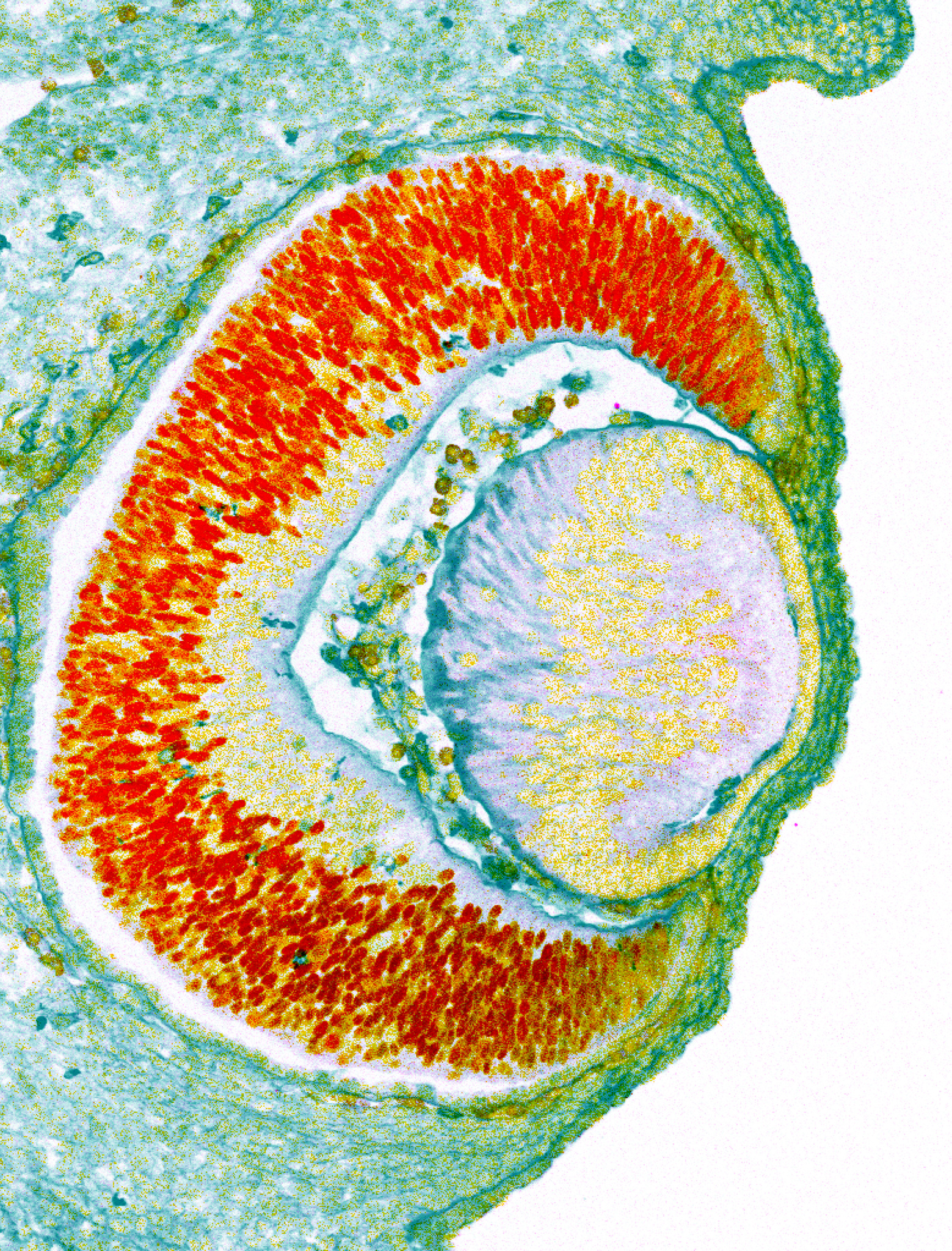
Oliver was the senior author of a Cell Reports publication, which used stem cells and induced pluripotent stem cells to generate eye organoids that mimic early eye development, as a tool to characterize molecular events regulating the formation of this complex organ.
The lead author was Nozomu Takata, PhD, a postdoctoral fellow in Oliver’s lab. Other Northwestern Medicine authors included Luciano Fiore, PhD, Sandra Acosta, PhD, and Hyea Jin Gil, PhD; all postdoctoral fellows in Oliver’s lab.
Oliver is also a professor of Medicine in the Division of Nephrology and Hypertension, and the director of the Center for Vascular and Developmental Biology at the Feinberg Cardiovascular Research Institute.
Recent advances in 3-D stem cell cultures facilitated the use of lab models that closely mimic actual tissue development, allowing scientists to better understand the molecular and morphogenetic processes underlying organ development. This study looked at the neuroretina, a collection of eye cells that help convert light into neural signals.
Using these tools, the scientists identified a gene — Rspondin-2 — as a critical player in mammalian neuroretina differentiation, according to Oliver.
The authors found that during early development, when the eye is initially forming and is still just an outgrowth of neural tissue, the activity of the transcription factor Six3 is required to repress Rspondin-2 from the anterior part of the head where eventually the eyes are going to form.
“The prospect of using stem cell-based therapies to treat different types of retinal diseases is becoming a real possibility; therefore, having a better understanding of the cellular and molecular processes controlling eye morphogenesis and neuroretina differentiation is critical,” Oliver said.
Further, the study showed using stem cells can accurately recreate live organs in lab-grown models, according to Oliver, opening up possibilities for future research.
“Our results further validate the organoid culture system as a reliable and fast alternative to identify and evaluate genes involved in eye morphogenesis and neuroretina differentiation in vivo,” Oliver said.
Uncovering the genetics of glaucoma symptoms
Oliver also collaborated on a paper published in the Journal of Clinical Investigation, where the authors correlated alterations in one gene with glaucoma, uncovering a potential treatment pathway.
The paper identified elevated retinal pressure and impairment of cell function in mouse models when the gene angiopoietin (Angpt) and its receptor, Tie2, were turned off. The investigators traced the symptoms to a weakened pressure-relieving vessel called Schlemm’s canal. Surprisingly, when they reactivated Tie2, it rejuvenated the vessel, suggesting a possible therapeutic pathway for glaucoma treatment, according to the study.
Glaucoma is a leading cause of blindness in the United States, involving the death of retinal cells caused by chronic elevated intraocular pressure. In healthy people, fluid builds up around the eye and drains through the Schlemm’s canal or through intracellular spaces, but the majority of glaucoma patients experience insufficient drainage — causing elevated pressure.






