Northwestern Medicine scientists showed how the microRNA-103/107 family (or miRs-103/107) regulate aspects of biological processes in the stem cell–enriched limbal epithelium of the eye.
Published in The Journal of Cell Biology, the study links for the first time the cellular processes of autophagy and macropinocytosis. Cells invoke autophagy, or “self-eating,” as a way to dispose of waste and as a response to stress. In macropinocytosis, cells take in large gulps of material including fluids, membrane, bacteria and viruses.
“We demonstrated that miRs-103/107 are important for the proper regulation of the end-stage autophagy and preventing excessive macropinocytosis,” said lead author Robert Lavker, PhD, Jack W. Graffin, M.D. Research Professor and professor of Dermatology.
Previously, Lavker and his team have found this microRNA family to be preferentially expressed in the limbal epithelium, which houses stem cells that maintain the corneal epithelium. This miRNA family helps regulate the limbal epithelial basal cells’ ability to divide and maintains the extensive proliferative capacity of these cells.
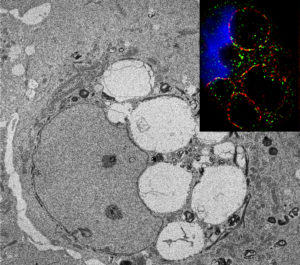
Co-first authors Han Peng, PhD, research assistant professor of Dermatology and Jong Kook Park, PhD, a post-doctoral fellow in Dermatology, silenced miRs-103/107 and observed large vacuoles developing in the limbal epithelium due to macropinocytosis.
Normally, after a cell gulps material and ingests it, the large vacuoles that are formed are recycled; however, in cells depleted of miRs-103/107, the macropinocytotic-derived vacuoles remained within the cells.
To further understand why these vacuoles persisted in cells, the scientists collaborated with Josh Rappoport in the Nikon Imaging Center and used super-resolution SIM microscopy to observe the morphology of the vacuoles. They found surface markers on the vacuoles that were associated with autophagy. Through further testing, they demonstrated that the vacuoles remained in the cell due to a defect in dynamin localization leading to a failure in the end stages of autophagy.
In future research, Lavker and his team plan to study how autophagy affects stem cell populations and how macropinocytosis functions in the normal corneal epithelium. They will also investigate how these processes are altered during wound healing and in diseases of the corneal epithelium such as dry eye and diabetes.
“We are the first investigators to study the basic mechanisms underlying these processes in the corneal/limbal epithelia,” Lavker said. “This work will lay the foundation for a whole investigative journey into both autophagy and macropinocytosis.”
Lavker is a member of the Center for Genetic Medicine and Robert H. Lurie Comprehensive Cancer Center of Northwestern University. Peng, Park and Lavker are members of the Northwestern University Skin Disease Research Center. CongCong He, PhD, assistant professor of Cell and Molecular Biology, and post-doctoral fellows Nihal Kaplan, PhD, Wending Yang, PhD, and Ying Dong, PhD, were also collaborators on the study.
This study was funded by National Institutes of Health grants EY06769, EY017539 and EY019463 and DK094980, National Natural Science Foundation of China grant 31300814, a Dermatology Foundation research grant and Career Development Award, and a MidWest Eye Bank research grant.