Too many dendritic cells – important messengers in the immune system – can lead to autoimmune disease. But what happens when the body doesn’t have enough of these critical immune cells?
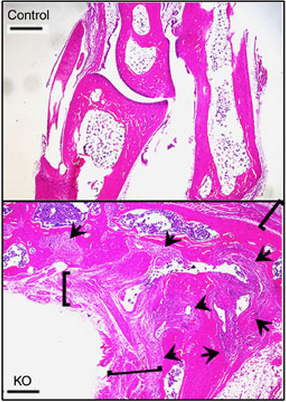
A new Northwestern Medicine study published in Nature Communications found that reducing dendritic cells in mice leads to inflammatory arthritis. These findings will help scientists investigate the pathogenesis of rheumatoid arthritis, a chronic autoimmune disease of the joints in which inflammation destroys cartilage and bone, causing pain and severe disability.
“Dendritic cells are professional antigen-presenting cells that are critical for mounting an immune response during immunization or against invaders such as bacteria and other microbes,” said senior author Richard Pope, MD, Mabel Greene Myers Professor of Medicine and chief of the Division of Medicine-Rheumatology.
Dendritic cells present antigens on their cell surface to activate T-cells, which help immune cells called B-cells produce antibodies that attack pathogens.
In the study, Dr. Pope and colleagues including first author Qi-Quan Huang, MD, research associate professor in Medicine-Rheumatology, developed a mouse model deficient in the Flip gene, which encodes a protein that protects against cell death. The scientists anticipated that the number of dendritic cells would be reduced without the protein that normally prevents their death.

“To our surprise, these mice developed a spontaneous arthritis,” Dr. Pope said. “The mice were deficient primarily in one subset of dendritic cells that is known to be important for the development and maintenance of regulatory T-cells.”
The mice also had less of those regulatory T-cells, which are critical for suppressing autoreactive T-cells and the formation of autoantibodies – cells and antibodies that attack one’s own body. Transferring additional regulatory T-cells into the arthritic mice improved their arthritis symptoms and inhibited the autoreactive T-cells.
The scientists will continue to use the new mouse model to explore the link between rheumatoid arthritis and the loss of regulatory T-cells.
“Future studies employing these mice will allow us to understand how systemic autoimmunity can be directed against the joints and how dendritic cells and regulatory T-cells might be manipulated to treat rheumatoid arthritis more effectively with less risk,” said Dr. Pope, who is a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. “We also want to find out how the disease might be prevented when the first signs of autoimmunity are detected.”
This study was supported by National Institute of Health (NIH) grants AR048269, AR065076, AR064464, HL108795, AR050250, AR054796, AR055503, AI067590, AI041985, AI079056, AI108634, AR006634 and AR059754; the Solovy Arthritis Research Society; American College of Rheumatology, Rheumatology Research Foundation; the Israel Binational Foundation; by the Northwestern University Mouse Histology and Phenotyping Laboratory; and National Cancer Institute grant CA060553.