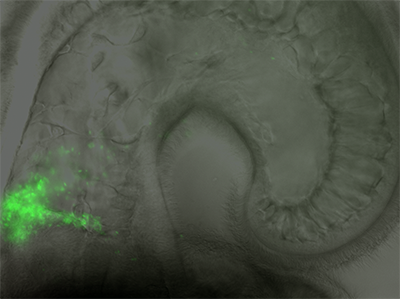
Through studying an organ that naturally has only a single bacterial resident in the Hawaiian bobtail squid, Mark Mandel, PhD, assistant professor in Microbiology-Immunology, has found bacterial genes that may help to understand the mechanisms by which humans acquire beneficial microbes from the environment.
The paper, published Proceedings of the National Academy of Sciences, is the first to globally identify the Vibrio fischeri bacteria genes required for colonization and the way those bacteria establish colonies in the squid’s light organ: the researchers found about five times as many new factors as were known previously.
“If we had a better sense of what processes were required for this to happen in a robust and reproducible way, then when things go awry like in inflammatory bowel disease or skin conditions we’d be better prepared to design a remedy for it,” he said. “If you take probiotics for example, the current technology of probiotics is they don’t stay colonized and one of the reasons for this is we don’t understand the principles of colonization in the gut.”
Using a squid model illuminated the processes by which bacteria colonize animal hosts in each generation; it allowed Mandel to observe what the genes do in an intact animal and collect an atlas of the crucial factors that are required for host colonization. Mandel and his team mutated all of the genes in bacteria and observed which mutants survived in the squid. The scientists inferred that the genes mutated in the bacteria that were not found in the host were important for communication between the bacterium and host for colonization.
“Animals have a very toxic environment, for example, animals have anti-microbial peptides and reactive oxygen-nitrogen species,” said Mandel. “The bacteria that can thrive and survive in that environment are the ones that can go on and colonize the host.”
Mandel discovered that a chaperone protein, DnaJ, has an effect on biofilm development – a sticky aggregation of bacterial cells. This protein plays a role in a specific stage of bacterial development and may relate to how the animal allows the bacteria to respond. In future studies, Mandel aims to learn how the host and bacteria communicate to regulate the formation of biofilm.
“Biofilms are really prominent in chronic infections in humans: everything from dental plaque to cystic fibrosis in the lungs – across many conditions in which bacteria aggregate,” he said. “In a pathogenic setting, bacterial aggregation has terrible consequences and it is hard to address those aggregates with antibiotics or current medical technology. So asking how this communication occurs in an intact animal host is of great interest.”
The scientists also found that bacterial mutant defective in genes predicted to detoxify copper, an antimicrobial metal found in squid tissues, had trouble colonizing. They also characterized a secretion system that plays an important role during colonization. In future studies, Mandel plans to further investigate the role copper has in the establishment of bacterial colonies and how the protein secretion system impacts colonization.
The research was supported by National Science Foundation grant IOS-0843633, National Institutes of Health grant DK089121, National Science Foundation grant IOS-1258099 and National Institute of General Medical Sciences Cellular and Molecular Basis of Disease T32 training grant GM08061.






