Northwestern Medicine scientists have discovered that a specific type of white blood cell’s behavior may explain how rheumatoid arthritis develops.
Rheumatoid arthritis is a chronic autoimmune disease that affects joints, especially in the hands and feet. It creates inflammation that destroys cartilage and bone, causing pain and severe disability.
“The main culprit is an overactive immune system in patients with rheumatoid arthritis, whose failsafe mechanism has gone awry,” said Harris Perlman, PhD, Solovy/Arthritis Research Society Professor in Medicine-Rheumatology.
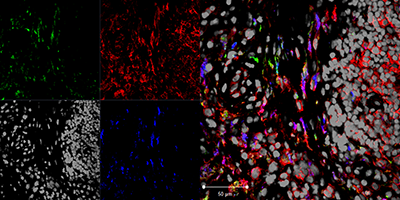
White blood cells called monocytes circulate in the bloodstream. When foreign substances like infection enter the body, some monocytes migrate to the infected tissue and become macrophages, white blood cells that protect the tissue. Previously, the specific role of monocytes and macrophages in the progression of arthritis has been unclear.
Perlman and Alexander Misharin, MD, PhD, research assistant professor in Rheumatology, examined the role of two types of macrophages: classically activated M1s, which encourage inflammation – the body’s normal response to fight off injury and foreign pathogens; and M2 macrophages, which repair the inflamed tissue afterward.

The scientists also studied the role of macrophages that are born on the body’s tissues instead of forming from monocytes recruited via the bloodstream. These tissue-resident macrophages have the M2 function – reducing inflammation caused in the early stages of tissue repair.
In rheumatoid arthritis, the immune system triggers inflammation to fight its own healthy tissues and, in the process, overwhelms the anti-inflammatory immune cells normally in charge of repair.
“Macrophages are central to the disease progression,” Perlman said. “Current therapies that target these cells help induce remission in patients. Unfortunately, very little is known about the genetic makeup of the monocytes that enter the joint during normal growth and during rheumatoid arthritis.”
Dual Role for Immune Cells Indicates Potential Target for Treatment
In a study published October 16 in Cell Reports, Perlman’s team showed that an influx of non-classical monocytes – the immune cells recruited to promote tissue healing in normal conditions – are essential to rheumatoid arthritis disease initiation and progression. Interestingly, the cells take on dramatically different roles throughout the course of disease.
Using a mouse model, the scientists simulated the disease’s three phases: initiation, propagation and resolution. They found that the non-classical monocytes expressed a complex set of both M1 and M2 genes. In the early stages of the disease, they promoted tissue injury – and the progression of arthritis – by inducing inflammation, overpowering the anti-inflammatory tissue-resident macrophages. But later, during the disease’s resolution, the same non-classical monocytes switched roles to initiate tissue repair, indicating a target for potential treatment.
“Therapies that target these macrophages and alter their profile will be extremely beneficial for rheumatoid arthritis patients,” said Perlman, a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
The findings in this study could apply broadly: Further research could determine whether non-classical monocytes contribute to inflammation and tissue injury in other autoimmune diseases.
Additional Feinberg authors include Carla Cuda, PhD and Rana Saber, both of Rheumatology, Sergejs Berdnikovs, PhD, ’12 GME, of Medicine-Allergy-Immunology, and Scott Budinger, MD, of Medicine-Pulmonary and Critical Care.
This research was supported by NIH grants AR050250, AR054796, AI092490 and HL108795, the Solovy/Arthritis Research Society and Arthritis Research UK grant 18547.






