In the wild, rabies virus spreads throughout the brain, almost always resulting in death. But in the hands of Steven DeVries, MD, PhD, and Yongling Zhu, PhD, the characteristic that makes it so deadly, its ability to move from neuron to neuron, is being harnessed for discovery.
As the only lab making the virus at Northwestern University Feinberg School of Medicine, DeVries and Zhu have grown rabies to be controlled like a vehicle at a red light, a process that would prevent it from spreading in humans if exposed.
In their versions, the viral gene for the protein that allows rabies to enter neurons and spread is removed. This protein is later reintroduced to the virus, allowing it to enter a single neuron, before stopping. If a second batch of the protein is added to the membrane of the infected neuron, the virus can take another step, before coming to a halt again. By monitoring how the virus progresses, the DeVries lab is working to discover the pathways signals take once received by the retina.
“Wild-type rabies has the ability to jump from one neuron to the next to the next, and has recently been harnessed for use in circuit tracing,” said DeVries, David Shoch Associate Professor of Ophthalmology. “The great theme here is we’re harnessing nature. Rabies has evolved over millennia to move across neurons and we’re taking it, and we’re adapting it.”
So far, DeVries and Zhu have generated more than 20 variants of rabies, each carrying a fluorescent protein, calcium sensor, or light-sensitive channel, that allows the scientists to see and manipulate the connections revealed by the virus as it progresses from neuron to neuron.
As one of the first groups to introduce rabies into the retina, DeVries and Zhu find themselves in a race for results with highly regarded labs in Basel, Switzerland, and at Harvard University. Although the virus has been used with success to map the wiring of other parts of the brain, not until recently has it been introduced to the light-sensitive tissue lining the inner surface of the eye.
“This is really unexpected for me,” DeVries said in regard to the direction of his lab. “I am trained as a neuroscientist and only had 10 virology lectures in medical school. Yet, half the work we do in the lab relies on viruses.”
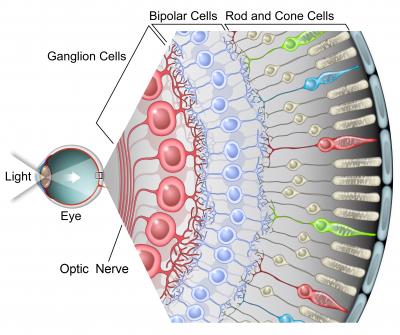
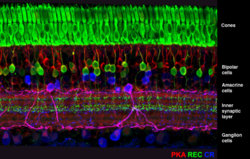
The circuitry of the retina is important because it mediates the first steps in vision and can act as a model for processing in other parts of the central nervous system (CNS). The retina is also easy to isolate and maintain in the lab. It contains more than 70 cell types, but each has been cataloged, a rarity for CNS regions.
“The first step in understanding how the retina processes visual information is to map out the connectivity within a circuit,” Zhu said. “Rabies tracer plays a crucial role in this mapping since it spreads from the ganglion cells, the output neurons of the retina, to the presynaptic neurons, the amacrine and bipolar cells that mediate the transformations between inputs and outputs.” The modified rabies allows the extent of the advancement of the virus to be controlled.
In DeVries’ lab, major advancements using the rabies tracing technique have been made but not published. What impedes the findings is the need to improve the procedure, and that’s what they are racing to achieve. His lab has seen many pairs of connected cells, but optimizing the technique will allow them to obtain enough data to firmly establish connectivity patterns.
“It’s really a global problem to try and understand which neurons talk to each other. A lot of time their processes are all mixed together. What’s nice about this tracer technique is that it reveals these connections, and you can put these florescent proteins into the virus and label and see as the virus moves across the synapses,” DeVries said.






