From neurons in an autism-causing condition to inflamed human lung tissue macrophage cells, compelling images illustrate a broad array of scientific findings this year. Here are a selection of eye-catching images from research published by faculty at Feinberg in 2014.
A slideshow of these images is also available.

Focusing on Synaptic Development in Fragile X Syndrome
Northwestern Medicine scientists identified events that may contribute to delayed synaptic and neuronal development in fragile X syndrome, the most common known cause of autism.
Anis Contractor, PhD, associate professor in Physiology, and a team of lab members led by postdoctoral fellow Qionger He, PhD, found that the normal maturation of the neurotransmitter GABA is delayed in fragile X mice.
This delay may prevent the proper development of cells within the brain’s cortex. The findings were published in the Journal of Neuroscience.
Read the full story here.

Iron May be at Heart of Cancer Drug’s Cardiotoxicity Effect
Scientists discovered the role iron plays in weakening a person’s heart following treatment with the common chemotherapy drug doxorubicin (DOX). Hossein Ardehali, MD, PhD, associate professor in Medicine-Cardiology and Pharmacology, found that cardiomyopathy, a prevalent side effect of DOX, is dependent on the accumulation of iron inside the mitochondria of cells.
Doxorubicin has been credited as a “wonder drug” for greatly reducing pediatric cancer deaths throughout the 1990s. Although heart damage remains the most serious side effect, DOX is commonly used in the treatment of a wide range of cancers. The findings were published in the Journal of Clinical Investigation.
Read the full story here.

Uncovering Selective Cell Death in Ataxia
Working with a newly created preclinical mouse model of ataxia, a neurological disorder characterized by loss of balance and coordination, Marco Martina, MD, PhD, associate professor in Physiology, wondered why the Purkinje neurons linked to the condition persisted well past the onset of disease.
Closer inspection revealed that although the neurons had not died, they had been altered as early as a few weeks of age. It also uncovered that a different neuron population, unipolar brush cells (UBC), had been completely eliminated within a month of disease onset.
Both changes were caused by the mutation of ion channel TRPC3, a known factor in the development of the disorder. These findings were published in the Journal of Neuroscience.
Read the full story here.

Discovering a New Gene with Role in Inflammation
Northwestern Medicine scientists found a new gene and revealed its role in the body’s inflammatory response against infection.
“In today’s scientific climate it’s not common to make a discovery like this because the human genome has been largely mapped,” said Christian Stehlik, PhD, John P. Gallagher Research Professor of Rheumatology. “Somehow, this gene and the protein it encodes had been overlooked,” Stehlik said.
The work by Stehlik and colleagues may provide a new therapeutic target against inflammatory responses.
These responses can be produced when the body recognizes foreign DNA from a virus like HIV, or when it attacks itself in autoimmune diseases such as Systemic lupus erythematosus, inflammatory myocarditis or Aicardi-Goutières syndrome.
The findings were published on Feb. 16 in Nature Immunology.
Read the full story here.

Protein May Provide Early Biomarker for Prostate Cancer
Elevated levels of a protein associated with cell cycle regulation may allow physicians to identify prostate cancer at its earliest stages.
Debabrata Chakravarti, PhD, professor in Obstetrics and Gynecology-Reproductive Biology Research, led the Northwestern Medicine study, which revealed the surprising role for WDR5.
“Our results showed that the overexpression of this protein may be necessary for the initiation, maintenance and growth of prostate cancer,” Chakravarti said. “If WDR5 is in fact a driver protein, we may also be able to develop a compound that would block a critical interaction and stymie the disease.”
Prostate cancer is the second-leading cause of cancer deaths in North American men. In the United States, more than 225,000 are expected to be diagnosed with the disease this year and nearly 30,000 will die.
The findings were published in Molecular Cell.
Read the full story here.

Investigating Spiral Formation in the Cornea
As some mammals mature, cells in the cornea’s epithelium tend to develop patterns such as spirals.
Learning how these spirals form and determining their function can help to understand how the human eye develops and give insight into some of the diseases affecting corneal development, as was seen in a recent Northwestern Medicine study.
Philip Iannaccone, MD, PhD, George M. Eisenberg Professor of Pediatrics, proposed a framework for explaining the development of the spiral pattern on the cornea in a recently published paper in Biomechanics and Modeling in Mechanobiology. The research was also published in the journal Complexity.
“In disease states this spiral pattern is disrupted, and we want to know what elements could be causing disease,” Dr. Iannaccone said.
Read the full story here.
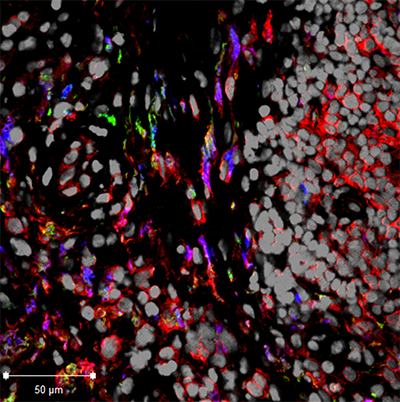
Specific White Blood Cells May Induce Rheumatoid Arthritis
Northwestern Medicine scientists discovered that the behavior of a specific type of white blood cell may explain how rheumatoid arthritis develops. Rheumatoid arthritis is a chronic autoimmune disease that affects joints, especially in the hands and feet. It creates inflammation that destroys cartilage and bone, causing pain and severe disability.
“The main culprit is an overactive immune system in patients with rheumatoid arthritis, whose failsafe mechanism has gone awry,” said Harris Perlman, PhD, Solovy/Arthritis Research Society Professor in Medicine-Rheumatology. The findings of the study were published in Cell Reports.
White blood cells called monocytes circulate in the bloodstream. When foreign substances like infection enter the body, some monocytes migrate to the infected tissue and become macrophages, white blood cells that protect the tissue. Previously, the specific role of monocytes and macrophages in the progression of arthritis has been unclear.
Read the full story here.

Signs of ALS Detected in the Eye
Northwestern Medicine Scientists found evidence of ALS-related deposits in the eyes of a patient for the first time, opening a new potential avenue for diagnosing and tracking the disease.
In ALS, proteins accumulate into clumps in the motor neurons of the brain and spinal cord. The neurons stop working and eventually die, causing muscle weakness and impaired speaking, swallowing, breathing and death.
In this study, Amani Fawzi, MD, associate professor in Ophthalmology and collaborators studied the retina of a patient with the most common genetic form of ALS. These investigations identified the same protein deposits in the retina of the patient that are usually found in the brain in this type of ALS. The findings were published in Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration.
Read the full story here.






