
A Northwestern Medicine study has discovered that elevated PALI1 in advanced prostate tumors mediates crosstalk between two primary epigenetic silencing mechanisms, suggesting that dual epigenetic inhibition may be an effective therapeutic strategy, according to findings published in Molecular Cell.
“Our discoveries of PALI1’s critical role in bridging and exploiting two major epigenetic silencing machineries for gene repression and tumor promotion strongly advocate combinatorial use of their respective pharmacological inhibitors,” said Jindan Yu, PhD, professor of Medicine in the Division of Hematology and Oncology and senior author of the study.
A distinctive feature of many cancers is uncontrolled growth caused by dedifferentiation, which occurs when differentiated cells forget their identity and regain lineage plasticity. In previous work, PALI1 had been identified as an accessory protein of the Polycomb Repressive Complex 2 (PRC2), which maintains the pluripotency of stem cells by silencing genes essential for cell differentiation.
Core subunits of PRC2 are among the most strongly up-regulated genes in advanced prostate cancer. While several PRC2 genes are currently known to be upregulated in advanced prostate cancer, the role of PALI1, a newly discovered PRC2 accessary protein, had not yet been fully understood.
In the current study, the investigators used co-immunoprecipitation and genome-wide location analysis to identify novel protein interactions and intracellular protein binding mechanisms in metastatic prostate cancer cells and used xenograft models to study gene function in advanced prostate cancer tumors.
They discovered not only that PALI1 was upregulated, but the protein also competes with the JARID2 protein for binding to PRC2 and directly interacts with the G9A protein, driving cell proliferation and invasion.
Notably, PALI1 bridges a unique interaction between PRC2 and G9A proteins, both of which play a role in the epigenetic silencing of a set of genes called G9A-PALI1-SUZ12 (GPS), which are involved in cell differentiation and are suppressed during tumorigenesis.
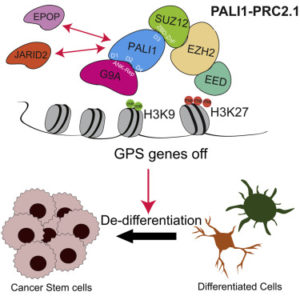
“PALI1 thus strongly suppresses differentiation genes, induces cancer cell stemness and promotes tumor growth,” said Yu, who is also a professor of Biochemistry and Molecular Genetics and a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
The loss of GPS genes is associated with poor clinical outcomes, and targeting PRC2 and G9A using complimentary inhibitors may have therapeutic potential, according to Yu.
“Fortunately, the GPS can be rescued through simultaneous targeting of PRC2 and G9A using their respective inhibitors, a therapeutic approach that might benefit a large number of patients with advanced prostate cancer,” Yu said.
Moving forward, Yu said her team aims to characterize other critical functions of PALI1 in prostate cancer and in other cancer types, findings that she said can drive bench-to-bedside translation.
“Our study is the first to dive into the roles of PALI1 in cancer. We are also among the first to study PALI1 in the human setting, as the limited studies on PALI1 are mostly on mouse embryonic stem cells,” Yu said. “We developed reagents for human PALI1 genes and proteins that will be useful for the investigation of PALI1 in various human diseases.”
Co-authors of the study include Changsheng Zhao, MD, associate professor of Medicine in the Division of Hematology and Oncology, and Ali Shilatifard, PhD, the Robert Francis Furchgott Professor and chair of Biochemistry and Molecular Genetics.
This work was supported in part by the American Cancer Society grant IRG-18-163-24, National Institutes of Health grants P20GM121327 and R03CA256230, prostate SPORE grants 5P50CA180995, R50CA211271, R01CA172384 and R01CA227918, and Department of Defense grants PC160328 and PC160856.






