A multidisciplinary team of investigators has engineered a more accurate model for studying the mechanisms underlying atrial fibrillation and its response to treatment, according to findings published in Science Advances.

Atrial fibrillation (AF) is the most common type of cardiac arrhythmia, causing a quivering and irregular heartbeat, which can lead to blood clots, stroke and heart failure. According to the American Heart Association, more than 12 million Americans are projected to be diagnosed with atrial fibrillation by the year 2030.
Patients with AF can be treated through a combination of lifestyle changes and antiarrhythmic drugs, which block ion channels in the heart muscle to help regulate the beating of the heart.
However, response to treatment can vary among patients and approximately half of patients will experience recurrent AF shortly after treatment. This is in part due to a lack of understanding of the genetic mechanisms of AF, according to the authors.
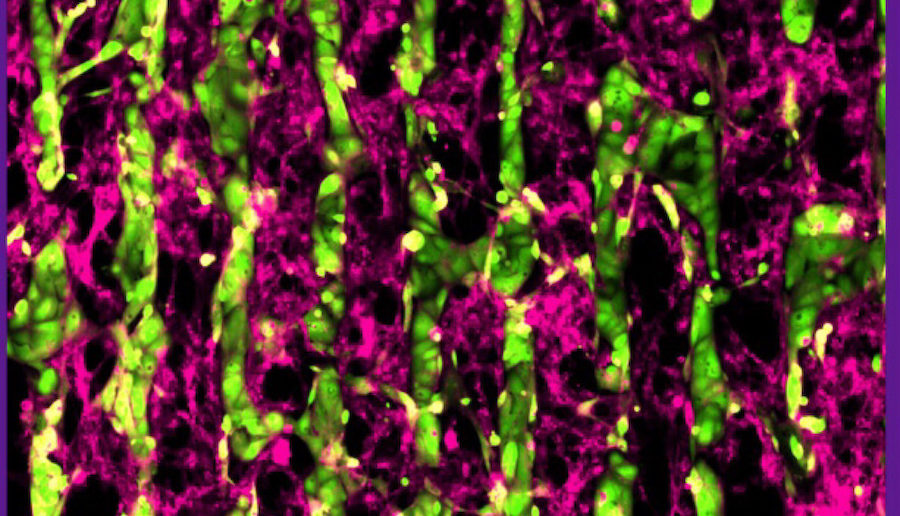
In the current study, investigators at the University of Illinois Chicago and Northwestern engineered and studied mature atrial cardiomyocytes from induced pluripotent stem cells (iSPCs). This approach aimed to address an ongoing challenge in the field, which involved generating atrial cells that closely mirror cardiomyocytes from an actual human heart, said Carlos Vanoye, PhD, research professor of Pharmacology and a co-author of the study.
“In this particular study, iPSC-derived atrial cardiomyocytes were co-cultured with cardiac fibroblasts and this allowed the cells to better acquire characteristics that we associate with mature atrial cardiomyocytes, a better system to study different therapeutic approaches,” said Carlos Vanoye, PhD, research professor of Pharmacology and a co-author of the study.

Specifically, the investigators developed a protein patterning process within multi-well plates to engineer co-cultures containing iPSC-atrial myocytes and fibroblasts, which significantly enhanced the atrial myocytes’ structural, electrical, contractile, and metabolic maturation. Feinberg investigators specifically demonstrated the electrophysiological properties of the cells using an advanced automated system.
“Overall, the co-cultured cells were more mature than cells not grown in the presence of fibroblasts,” Vanoye said.
According to the authors, this approach can help investigators better understand cell signaling in the atria, as well as help test the efficacy of current and future AF therapies.
“Because the co-cultured cells are more mature, they can be more responsive to drugs,” Vanoye said.
Al George, Jr., MD, chair and the Alfred Newton Richards Professor of Pharmacology, was a co-author of the study.
This work was supported by the National Institutes of Health grants R01 HL150586, R01 HL148444, and VA Merit Review grants 1IO1BX004268, T32 HL139439 and 1IO1BX004268.