
Northwestern Medicine investigators have discovered that the overexpression of a transcription factor prevented bone loss in mouse models of chronic kidney disease, according to a recent study published in the Journal of Clinical Investigation.
The findings demonstrate that the transcription factor hepatocyte nuclear factor 4 alpha (HNF4A) regulates bone frailty and is implicated in the development of renal osteodystrophy (ROD), a common complication of chronic kidney disease that decreases bone cell metabolism and weakens the bones over time.
“This manuscript provides a template for new discoveries that could help solve the underlying mechanisms of ROD,” said Nicolae Valentin David, PhD, the Frank Krumlovsky, MD, Professor of Medicine in the Division of Nephrology and Hypertension and senior author of the study.
More than 37 million people in the U.S. have chronic kidney disease, and most will also develop ROD. There is no cure for the condition except for a kidney transplant, and side effects can include bone loss, skeletal deformities, increased risk of bone fractures and cardiovascular issues.
Currently, treatments for ROD focus on treating systemic mineral metabolism disorder, which involves the correction of circulating phosphate, parathyroid hormone and vitamin D levels. Despite this and other current therapies, however, chronic kidney disease-related bone fractures in adult and pediatric patients have more than doubled over the last two decades due to the lack of ROD pathogenesis research, according to David.
The “turnover, mineralization, volume” (TMV) bone classification system used to define ROD type reflects that issue, according to Valentin. However, the current, limited understanding of ROD pathobiology doesn’t reflect the greater state of science in skeletal biology overall and in the understanding of bone cell signaling, he said.
“Lack of progress in improving ROD clinical outcomes despite strategies that consider the TMV classification support the need for treatments that are based on state-of-the art science in ROD pathobiology,” Valentin said.
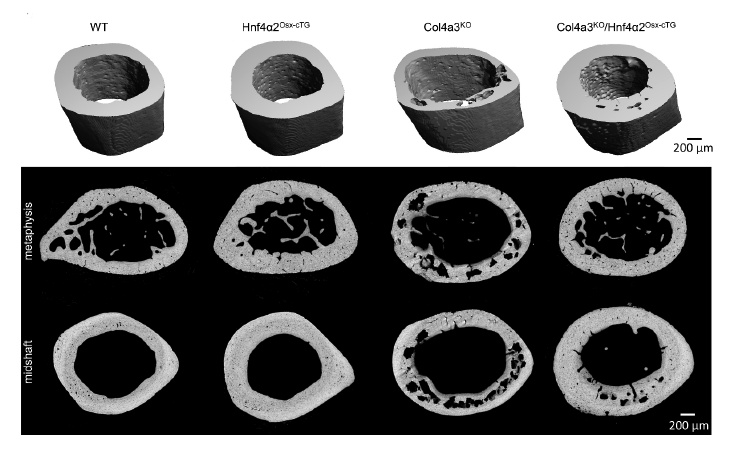
In the current study, Valentin’s laboratory investigated bone tissue samples from healthy patients and patients diagnosed with chronic kidney disease, and bone tissues samples from mouse models of ROD. Using RNA sequencing, they found that HNF4A, a transcription factor known to be expressed in the liver, is also expressed in bone and that HNF4A bone expression was reduced in patients and mice with ROD.
Next, the investigators overexpressed HNF4A in osteoblasts (cells that build bone) in mice with chronic kidney disease, finding that this decreased bone frailty and overall corrected skeletal abnormalities.
Using multi-omics techniques to analyze bone tissue and osteoblasts lacking or overexpressing HNF4A isoforms, they discovered that the isoform HNF4A2 regulates osteogenesis, bone cell metabolism and cell death.
“What is particular about the role of HNF4A2 in bone is its conserved function across organs and cells, to regulate metabolic activity and transport in osteoblasts comparable to its role in hepatocytes and proximal tubular kidney cells together with the regulation of osteoblast-specific osteogenic genes,” David said.
The findings suggest that ROD patients could potentially benefit from novel therapies targeting HNF4A expression to delay the progression of bone frailty and improve quality of life, according to David, but only if more research efforts focus on this goal.
“State-of-the art methods in omics applied to bone will lead to major advances in our understanding of ROD pathogenesis and inform the development of targeted, disease-modifying and curative strategies. These novel methods are only now being applied to ROD, but the fundamental infrastructure to develop patient-centered clinically relevant research is lacking, impeding the development of strategies that will improve the lives of patients living with chronic kidney disease,” David said.
David added that his team is now investigating the role of HNF4A2 in bone and kidney and its larger role in ROD progression and chronic kidney disease.
“We think that its progressive decline in bone which parallels the progression of chronic kidney disease is part of larger multi-organ metabolic reprogramming which, if prevented, may delay not only the progression of ROD but also the decline in kidney function,” David said.
Aline Martin, PhD, associate professor of Medicine in the Division of Nephrology and Hypertension, was a co-author of the study.
This study was supported by the National Institute of Health grants R01DK102815, R01DK114158, R01DK101730, R01DK131046 and R56DK127986.






