Spherical nucleic acids, structures made from short snippets of DNA radially arranged around a nanoparticle core, have the ability to travel to different organs in the body depending on the type of hydrophobic “tail” used to anchor it to the particle surface, according to a recent Northwestern study published in the journal ACS Nano.
These nanoparticles can target genetic drivers of disease-causing proteins, so these findings give scientists a broad template for exploring organ-specific therapies, according to Chad Mirkin, PhD, professor of Medicine in the Division of Hematology and Oncology, the George B. Rathmann Professor of Chemistry at Northwestern’s Weinberg College of Arts and Sciences, and senior author of the study.
“How these spherical nucleic acids are made dictates where they go and what organs they access,” said Mirkin, who is also a member of the Robert H. Lurie Comprehensive Cancer Center at Northwestern University. “Once you understand this, you realize that there is an enormous amount of design space.”
While nucleic acids in linear form do not readily enter cells, the equivalent sequence arranged in an SNA architecture will be rapidly internalized by cells. This uptake is likely due to SNAs’ greater physical resemblance to molecules called G-quadraplexes that are often internalized by cells.
“You can move constructs into cells and you can use that capability to make measurements in cells, or you can use such structures to regulate what goes on in the cell,” Mirkin said. “The latter is how we develop therapeutics.”
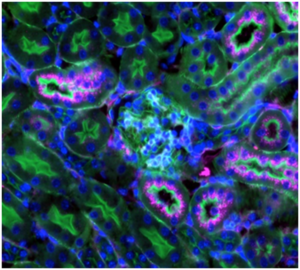
In the current study, investigators compared SNAs with two different types of “tails” on the ends of short DNA strands: a cholesterol tail and a lipid tail. The difference between each DNA “tail” was its ability to bind to the particle surface and interact with neighboring tails, which determines the release rate of the DNA sequence from the SNA’s nanoparticle core. Injecting these SNAs into mice, the scientists found SNAs with cholesterol tail DNA accumulated in greater amounts in the lungs, while SNAs with lipid tail DNA accumulated in greater amounts in the kidneys. These differences in DNA distribution highlight how changing the release rate of DNA from the SNA’s nanoparticle core can dictate what organs and cells the associated DNA reaches, according to Mirkin.
“Depending on whether you want to treat kidney disease or you want to treat lung disease, different constructs can be designed to yield greater or less accumulation,” Mirkin said.
Mirkin noted that the behavior of kidney-specific SNAs has sparked discussion with Northwestern Medicine nephrologists about future collaboration on therapies.
“With the conventional constructs that didn’t go into the kidney, that would be a silly choice,” Mirkin said. “Until you do this study, you don’t know how to design spherical nucleic acids to fully maximize their potential in a given organ of interest.”
Mirkin noted that adding these tails are not the only modification needed to deliver SNAs to a specific organ. Sending SNAs to the lungs could be enhanced through the use of a nebulizing spray to coat the lungs, while kidney-focused SNAs would need to be designed to get “stuck” in the kidney’s filtration system to enable uptake of theraptutics, because the nanoparticles are too big to enter the kidneys.
In any case, these discoveries, in conjunction with other recent studies demonstrating that tweaking SNA structure can direct them to enter the brain or penetrate through the skin, present Mirkin and his cast of collaborators with a wealth of opportunity. To sift through these options, Mirkin said he is now developing high-throughput screening methods to codify and test the “design rules” for SNAs, to establish a database of characteristics and materials from which organ-specific SNAs could be designed.
“If we want to optimize them for the kidney or the brain, what are the specific features that will get them there?” Mirkin said. “You have to reduce the number of shots on goal required, so now we’re creating assays that will do just that.”
Jason Werthheim, MD, PhD, the Edward G. Elcock Professor of Surgical Research and vice chair for research in the Department of Surgery, and Zheng Zhang, MD, research professor of Surgery in the Division of Organ Transplantation, were both co-authors of the study.
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health grants U54CA199091, R01CA208783 and P50CA221747