Study pinpoints brain region responsible for placebo response to pain.
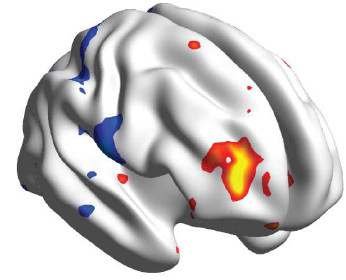
Scientists have identified for the first time the region in the brain responsible for the “placebo effect” in pain relief, when a fake treatment actually results in substantial reduction of pain, according to new research from Northwestern Medicine and the Rehabilitation Institute of Chicago (RIC).
Pinpointing the sweet spot of the pain-killing placebo effect could result in the design of more personalized medicine for the 100 million Americans with chronic pain. The fMRI technology developed for the study, published in the journal PLOS Biology, has the potential to usher in an era of individualized pain therapy by enabling targeted pain medication based on how an individual’s brain responds to a drug.
The finding also will lead to more precise and accurate clinical trials for pain medications by eliminating individuals with high placebo response before trials.
The scientists discovered a unique brain region within the mid-frontal gyrus that identifies placebo pill responders in one trial and can be validated (95 percent correct) in the placebo group of a second trial.
“Given the enormous societal toll of chronic pain, being able to predict placebo responders in a chronic pain population could both help the design of personalized medicine and enhance the success of clinical trials,” said Marwan Baliki, ’09 PhD, assistant professor of Physical Medicine and Rehabilitation and research scientist at RIC.
Baliki and A. Vania Apkarian, PhD, professor of Physiology at Feinberg in whose lab the research was conducted, are both corresponding authors on the paper.
Using drugs to treat patients’ pain has been trial and error, with physicians changing dosage or trying another type of drug if one doesn’t work.
“The new technology will allow physicians to see what part of the brain is activated during an individual’s pain and choose the specific drug to target this spot,” Apkarian said. “It also will provide more evidence-based measurements. Physicians will be able to measure how the patient’s pain region is affected by the drug.”
Currently, placebo response is primarily studied in healthy subjects within controlled experimental settings. While such experiments aid understanding of the biological and behavioral underpinning of placebo response in experimental (applied) pain, they translate poorly to the clinic, where pain is mainly chronic in nature, Baliki said.
In this new study and for the first time, scientists used functional magnetic resonance imaging (fMRI) combined with a standard clinical trial design to derive an unbiased brain-based neurological marker to predict analgesia associated with placebo treatment in patients with chronic knee osteoarthritis pain. Scientists showed placebo pill ingestion is associated with a strong analgesia effect, with more than half of the patients reporting significant pain relief.
If future similar studies can further expand and eventually provide a brain-based predictive best-therapy option for individual patients, it would dramatically decrease unnecessary exposure of patients to ineffective therapies and decrease the duration and magnitude of pain suffering and opioid use, Baliki and Apkarian said.
Other Northwestern authors include Thomas Schnitzer, MD, PhD, professor of Physical Medicine and Rehabilitation; Pascal Tétreault, PhD; and Etienne Vachon-Presseau, PhD.
The study was supported by grant NS035115 from the National Institute of Neurological Disorders and Stroke and grant AT007987 from the National Center for Complementary and Integrative Health, both of the National Institutes of Health. The Canadian Institutes of Health Research and Eli Lilly Pharmaceuticals also supported the research.






