Northwestern Medicine scientists have shed light on a hallmark of Parkinson’s disease that has been poorly understood up until now, the buildup of a protein called α-synuclein in the brain.
In a study published in Proceedings of the National Academy of Sciences (PNAS), they revealed a pathway for potential future therapies to treat the incurable neurodegenerative disease and disorders related to it. Currently, physicians are able to treat only the symptoms of Parkinson’s disease, because there are no disease-altering therapies available.
“What happens after α-synuclein starts accumulating? Why is it causing dysfunction of human neurons, and how can we prevent this dysfunction early in the disease process? This paper addresses these important questions,” said the study’s principal investigator Dimitri Krainc, MD, PhD, Aaron Montgomery Ward Professor and chair of Neurology.
Krainc and first author Joseph Mazzulli, PhD, assistant professor of Neurology, showed in the study that too much α-synuclein reduces the capacity of lysosomes, recycling factories in cells that remove cellular debris. The scientists found that this impairment occurs because enzymes in lysosomes called hydrolases, which digest that debris, are disrupted.
“Hydrolases have to be transported into the lysosome from the endoplasmic reticulum, and that transport is tightly regulated,” Krainc explained. “This trafficking is what the α-synuclein impairs. When these enzymes don’t make it to lysosomes, lysosomes can’t do their job.”
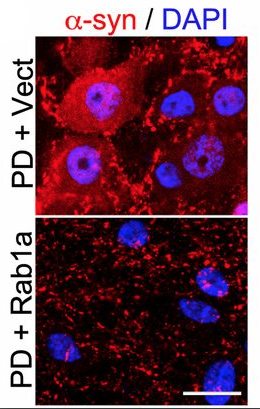
Using neurons acquired from patients with Parkinson’s disease by reprogramming their skin cells, the scientists demonstrated multiple strategies to restore hydrolase trafficking and thus improve lysosome function. These strategies included knocking down α-synuclein and overexpressing the protein rab1a, which is also involved in hydrolase transport.
By culturing human neurons over hundreds of days, the scientists were able to monitor the lysosomal system as the cells aged.

“We realized that this trafficking defect is an early event in the disease, and if we reverse it we can rescue these neurons from degenerating,” Krainc said. “Targeting this really critical downstream effect of accumulated α-synuclein may have therapeutic value. We are exploring this by developing small molecules as potential therapeutics.”
In another recent paper, published and featured in the Journal of Neuroscience, Krainc’s lab continued to illuminate the pathology of Parkinson’s disease. The scientists showed that mutations in the parkin gene – responsible for inherited forms of the disease – disrupt lysosomal trafficking pathways by reducing the function of the protein rab7.
“Studies of rare genetic forms of Parkinson’s disease such as parkin provide important insights and specific therapeutic targets for more common sporadic forms the disease,” Krainc said.
This research was supported by the National Institute of Neurological Disorders and Stroke grants R01NS076054, R01NS092823 and U24NS078338.






