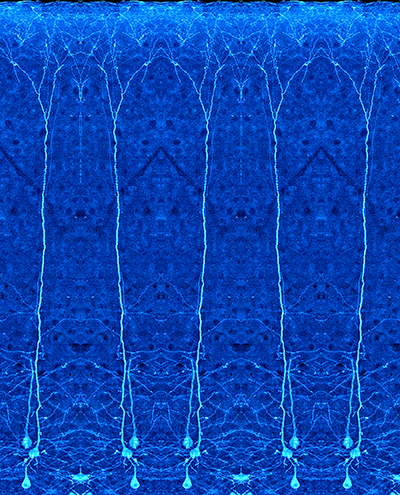
For the first time, scientists have revealed a mechanism underlying the cellular degeneration of upper motor neurons, a small group of neurons in the brain recently shown to play a major role in ALS pathology.
ALS, or amyotrophic lateral sclerosis, is a fatal neuromuscular disorder marked by the degeneration of motor neurons, which causes muscle weakness and impaired speaking, swallowing and breathing that leads to paralysis and death. Defects in upper motor neurons, which send messages from the brain to the spinal cord to activate voluntary movement, may be a starting point for the disease.
In a new study supported by the Les Turner Foundation, published January 16 in Cerebral Cortex, Northwestern Medicine scientists begin to explain why upper motor neurons are vulnerable to degeneration. They developed a new mouse model for studying these cells and found that increased stress in the endoplasmic reticulum (ER) is one culprit of upper motor neuron death.
“Now that we appreciate the importance of upper motor neurons, we need to develop therapies that improve their survival,” said principal investigator Hande Ozdinler, PhD, assistant professor of Neurology. “This study gives us a target to go after, bringing us one step closer to building effective treatment strategies.”
The new model features mice without UCHL1 protein function – mutations in UCHL1 gene have previously been implicated in motor defects in human patients. Using in vitro and in vivo methods, the scientists discovered that loss of UCHL1 protein function affects protein regulation pathways, ER stress and upper motor neuron survival.
“In this model, the timing and extent of upper motor neuron degeneration is unprecedented,” Ozdinler said. “All the other neurons in the brain remain healthy, which means that this model will be very useful for studying the health of the upper motor neurons.”
Upper motor neurons make up only about 150,000 of the billions of cells in the brain.
“In mathematical terms, they’re insignificant, but their function is so important. They act as the spokesperson of the brain by collecting, integrating, translating and transmitting brain’s message to the spinal cord targets, and by doing so they initiate and modulate voluntary movement,” Ozdinler said.

Ozdinler’s lab has spearheaded research establishing that upper motor neurons are essential to ALS pathology. Previously, scientists thought that spinal motor neurons were more important in ALS pathology – that upper motor neuron death was a mere secondary effect. In 2012, her group showed that an early event in ALS is spine loss in the apical dendrites of upper motor neurons, where they make connections with other neurons in the brain. In 2013, the lab generated the first reporter line for upper motor neurons, to help scientists visualize them with a green fluorescent protein.
“Now that we have a model and reporter line, we have the tools to develop therapies directed at the upper motor neurons,” Ozdinler said. “Survival requirements of these neurons cannot be ignored in ALS and in other diseases in which voluntary movement is impaired.”
The findings of the study could have applications to other neurodegenerative diseases that may share ER stress as an underlying cause.
“Parkinson’s, Alzheimer’s and ALS are different branches of the same tree,” Ozdinler said. “Subpopulations of patients may be developing these diseases due to the same dysfunctional cellular pathways. Finding a therapy for the pathway could help all of these patients.”
Ozdinler is a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, and the Cognitive Neurology and Alzheimer’s Disease Research Center.
Javier Jara, PhD and Barış Genç, PhD were co-first authors of the study. Additional authors include Gregory Cox, PhD, Martha Bohn, PhD, professor emeritus of Pediatrics in the Division of Neurobiology, Raymond Roos, MD, Jeffrey Macklis, MD, and Emel Ulupınar, MD, PhD, adjunct instructor of Neurology.
This study was funded by National Institutes of Health (NIH) grant R01NS085161-01, the Les Turner ALS Foundation, Wenske Foundation, NUCATS, NIH/ National Institute of Neurological Disorders and Stroke grants NS49553, NS49553 and NS41590, NIH Mechanisms of Aging and Dementia Postdoctoral Training Program and ALSA Safenowitz fellowship.






