A nano-sized discovery by Northwestern Medicine scientists helps explain how bipolar disorder affects the brain and could one day lead to new drug therapies to treat the mental illness.
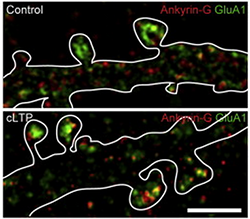
Scientists used a new super-resolution imaging method – the same method recognized with the 2014 Nobel Prize in chemistry – to peer deep into brain tissue from mice with bipolar-like behaviors. In the synapses (where communication between brain cells occurs), they discovered tiny “nanodomain” structures with concentrated levels of ANK3 – the gene most strongly associated with bipolar disorder risk. ANK3 is coding for the protein ankyrin-G.
“We knew that ankyrin-G played an important role in bipolar disease, but we didn’t know how,” said Peter Penzes, PhD, professor in Physiology and Psychiatry and Behavioral Sciences and corresponding author of the paper. “Through this imaging method we found the gene formed in nanodomain structures in the synapses, and we determined that these structures control or regulate the behavior of synapses.”
The results were published in the journal Neuron.
High-profile cases, including actress Catherine Zeta-Jones and politician Jesse Jackson, Jr., have brought attention to bipolar disorder. The illness causes unusual shifts in mood, energy, activity levels and the ability to carry out day-to-day tasks. About 3 percent of Americans experience bipolar disorder symptoms, and there is no cure.

Recent large-scale human genetic studies have shown that genes can contribute to disease risk along with stress and other environmental factors. However, how these risk genes affect the brain is not known.
This is the first time any psychiatric risk gene has been analyzed at such a detailed level of resolution. As explained in the paper, Penzes used the Nikon Structured Illumination Super-resolution Microscope to study a mouse model of bipolar disorder. The microscope realizes resolution of up to 115 nanometers. To put that size in perspective, there are 1,000 nanometers in a micron, and there are 25,400 microns in one inch. Very few of these microscopes exist worldwide.
“There is important information about genes and diseases that can only been seen at this level of resolution,” Penzes said. “We provide a neurobiological explanation of the function of the leading risk gene, and this might provide insight into the abnormalities in bipolar disorder.”
The biological framework presented in this paper could be used in human studies of bipolar disorder in the future, with the goal of developing therapeutic approaches to target these genes.
Other Feinberg authors include Katharine Smith, PhD, Katherine Kopeikina, PhD, Jessica Fawcett-Patel, Katherine Leaderbrand, Ruoqi Gao, Britta Schurmann, PhD, Kristoffer Myczek, Jelena Radulovic, MD, PhD, and Geoffrey Swanson, PhD.
This work was supported by the National Institute of Mental Health grants R01MH071316, R01MH097216, R01NS071952 and R01MH078064 and Marie Curie Outgoing Postdoctoral Fellowship 302281.






