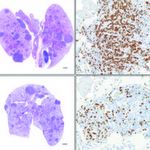
Here, the effects of treating preclinical non-small-cell lung cancer models with ATN-224 (lower two boxes) is seen in a reduction of tumor burden (dark purple) and cancer cells (brown).
Northwestern Medicine® scientists have demonstrated how inhibiting an antioxidant protein – which cancer cells depend on for survival – could produce an array of new cancer treatments. It’s a method opposite the long-standing approach of increasing antioxidants in cancer therapy.
Typically, reactive oxygen species (ROS) – oxidants – are constantly generated and eliminated within the body, as their buildup results in significant damage to cell structures. Cancer, however, uses them to maximize its ability to rapidly reproduce. To do this, they rely on intracellular antioxidants to keep ROS levels elevated but controlled.
Published in The Journal for Clinical Investigation, the preclinical research capitalizes on the fact that even cancer cells possess a vulnerability to ROS overload.
“There are of course a number of things to consider when inhibiting antioxidants, first among them is that the protein must be more selective, or vital, for cancer cells as compared to normal cells,” said Navdeep Chandel, PhD, professor in Medicine-Pulmonary and Cell and Molecular Biology, principle investigator of the project. “Also, the resulting increase in reactive oxygen species has to be high enough to result in cell death. If you don’t kill the cancer, it will thrive in this ROS-rich environment.”
With that in mind, Andrea Glasauer, PhD, considered the ramifications of inhibiting the antioxidant SOD1, a protein that accelerates the cells ability to eliminate superoxides.
The lab used in vitro and in vivo models of non-small-cell lung cancer (NSCLC) to test its hypothesis. NSCLCs represent more than 80 percent of all lung cancers, the leading cause of cancer death in the United States.
“As we began the project, a couple of fortuitous things occurred,” said Chandel, a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. “Having identified SOD1 as being somewhat dispensable in normal cells, a paper by Nobel Prize winner Harold Varmus and colleagues in the Proceedings of the National Academy of Sciences identified it as a potential target because of its overexpression in lung cancer.”
Navdeep Chandel, PhD, professor in Medicine-Pulmonary and Cell and Molecular Biology, has shown that by inhibiting antioxidants, it is possible to kill lung cancer cells.
The other fortuitous thing was collaborating with Andrew Mazar, PhD, research professor at Northwestern University’s Chemistry of Life Processes Institute and director of the Center for Developmental Therapeutics, who designed ATN-224, a drug that targets SOD1.
Chandel demonstrated that ATN-224 was able to reduce tumor burden in a mouse model of NSCLC. The findings offer proof that antioxidant inhibition has potential clinical applications as a single agent, or in combination with other drugs, for treatment of the disease.
“We never think a single agent will be the answer, so our future work will be in pursuing another target where the two could increase a treatment’s effectiveness,” Chandel said. “The goal is to hit a cancer cell all at once so that it flames out and dies. Targeting SOD1 along with other selective agents that reduce tumor burden might be an effective novel approach targeting cancer therapy.”
Laura Sena, a student in the Northwestern University Medical Scientist Training Program, and Lauren Diebold, a student in the Driskill Graduate Program in the Life Sciences, also contributed to the project.
The work was supported by the LUNGevity Foundation and the Consortium of Independent Lung Health Organizations convened by the Respiratory Health Association of Metropolitan Chicago; National Institutes of Health grants R01CA123067, F30ES019815 and T32-HL76139; a Dixon Translational Grant; and a Northwestern University Malkin Scholar Award.






