Jacek Topczewski, PhD, research associate professor of pediatrics, finds intrigue in the cells that make a face a face as we know it.
“When I got to Northwestern, I became interested in the role of the glypican family of proteins and the extracellular pathway that is implicated in cartilage organization,” Topczewski said. “Some people would suggest that this is a good model for cleft palete and cleft lip formation – one of the most common abnormal congenital defects in humans – so understanding this process may help us understand clefts.”
Affecting as many as one in 700 births in the United States, clefts are the result of abnormal facial development during gestation. The cleft, or gap, is the non-fusion of the body’s natural structures.

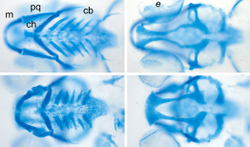
By monitoring the facial bone formation in zebrafish, Topczewski hopes to increase researchers’ understanding of human bone development. The fish, which are nearly translucent early in life, provide a window into every cell as it matures. Because a female fish will lay 200 or more eggs at once, another benefit is that thousands of offspring share similar genetic makeup.
“Our model predicts that glypican 4 is required for this very specific cell movement that helps shape the facial bones, by stacking of the chondrocytes – the healthy cells in cartilage. This stacking is very reminiscent of the growth plate in human bone,” Topczewski said. “One of the interesting parts about this is that if you look very closely at those cartilages, you’ll find that cells are normally organized in a neat way, like a stack of pennies, but when the function of glypican 4 is disrupted, chondrocytes are disorganized and defects occur.”
Topczewski is collaborating with researchers at the University of Louisville Birth Defects Center to investigate the genes involved in cleft palates. He has recently begun another coordinated project with Arun Gosain, MD, professor of plastic surgery, to develop a zebrafish model to study cranial suture formation. A premature fusion of sutures – craniosynostosis – is a relatively common birth defect. The goal of this work is to discover novel genes that control the timing of when sutures close.
Topczewski’s work on the glypican family of proteins was supported in part by National Institutes of Health grants R01DE016678, F32DE019058, and F32DE019986.






