Protein Essential for Hearing Also Vital for Pain Perception
The same protein that “translates” sound into nerve signals to the brain and enables individuals to hear is also required for pain perception, researchers from Northwestern University’s Feinberg School of Medicine have found.
Because the protein, TRPA1, is found in most—about 75 percent—of the body’s pain-perceiving neurons, but not in major organs, drugs that could block TRPA1 would be novel painkillers with few or no side effects, although targeting the inner ear may have to be avoided, said Jaime Garcia-Añoveros, PhD, who led the research.
Dr. Garcia-Añoveros, assistant professor of anesthesiology, neurology, and physiology at the Feinberg School, a member of the Northwestern University Institute for Neuroscience (NUIN), and a fellow of the Hugh Knowles Center for Hearing Research, and colleagues described the dual role of TRPA1 in the cover article of the April 20 issue of the Journal of Neuroscience. His co-authors, all members of his research group at Northwestern University, were Keiichi Nagata, PhD, and Anne Duggan, PhD, both research assistant professors of anesthesiology, and Gagan Kumar, PhD, postdoctoral fellow in anesthesiology.
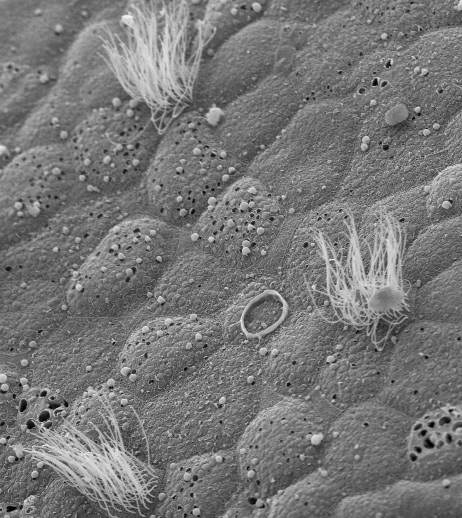
Dr. Garcia-Añoveros and his colleagues showed conclusive evidence that TRPA1, in addition to being expressed in nociceptors, or pain neurons, is present in the stereocilia of hair cells, the sensory part of the cells of the inner ear used for hearing as well as detecting gravity and maintaining balance.
Dr. Garcia-Añoveros and Dr. Duggan, also a NUIN researcher, initiated the study of TRPA1 in the late 1990s while searching for the channel that mediates hearing.
After they discovered that TRPA1 is expressed in the organ of Corti, the hearing organ of the inner ear, they were joined by colleagues at Harvard Medical School and Northwestern, and thus founded the groundbreaking group of scientists who in 2004 proposed that TRPA1 was a candidate for the mechanosensory channel of hair cells.
In the current study, the Northwestern researchers also demonstrated that TRPA1 channels have a unique combination of properties displayed by the hair cell transducer and by no other known channel. These similarities strongly suggest that TRPA1 is the pore that opens in response to sound, initiating the electrical signal cascade that ultimately reaches the brain as we perceive sounds.
The researchers have also found properties of the TRPA1 channel that account for its suspected parallel role in pain sensation, such as why in some cases pain from an injury will not go away as long as the injury remains.
Essentially, TRPA1 opens in response to painful stimulation, and ions enter the cell, making it less negatively charged, or depolarized. At this point most ion channels close, a phenomenon known as inactivation, because their signaling task has been achieved.
But TRPA1 senses the depolarization and responds to it by staying open; it will close only when the harmful stimulus goes away. However, if the depolarization is small, the TRPA1 channels close.
This means that TRPA1 could allow sensory neurons to ignore sustained innocuous stimuli (such as gentle pressure) but to respond to noxious stimuli (such as a pinch that causes tissue damage) and remain active as long as the noxious stimuli persist.
It also means that TRPA1 is sensitized by the opening of other nociceptor channels in the same sensory nerves; for example, those that respond to painful heat. This property of TRPA1 may thus account for painful phenomena in damaged tissues such as lack of desensitization or even some forms of enhanced sensitization, such as hyperalgesia (extreme sensitivity to pain) or allodynia (pain resulting from non-noxious stimuli to normal skin).
“Now we can look for TRPA1 channel blockers, which potentially will constitute novel analgesics that block pain at its initiation,” Dr. Garcia-Añoveros said.
“Such TRPA1 channel blockers should probably be used topically—preferably avoiding contact with the inner ear—but could also be applied systemically. In that case, the patient might have to put up with temporary deafness, a side effect that is probably better than extreme pain. What is critical at this point is to identify drugs or treatments that very specifically inhibit TRPA1,” he said.
TRPA1 is known to be activated by pain-producing chemicals such as the pungent components of edibles including wasabi, horseradish, mustard, cinnamon, and Listerine®, which explains why they sting the mouth.
This research was supported by grant RO1-NS044363 from the National Institute of Neurological Disorders and Stroke and grant R21-DC006089 from the National Institute on Deafness and Other Communication Disorders.
The full article is available online at http://www.jneurosci.org [25(16):4052-4061].
(Reprinted from the Northwestern University News Center.)